Summary
Scratching triggers skin flares in atopic dermatitis (AD). We demonstrate that scratching of human skin, and tape stripping of mouse skin, causes neutrophil influx. This influx in mice was largely dependent on the generation of leukotriene B4 (LTB4) by neutrophils and their expression of the LTB4 receptor BLT1. Allergic skin inflammation in response to epicutaneous (EC) application of ovalbumin to tape-stripped skin was severely impaired in Ltb4r1−/− mice, and required expression of BLT1 on both T cells and non-T cells. Co-transfer of WT neutrophils, but not neutrophils deficient in BLT1 or the LTB4 synthesizing enzyme LTA4H, restored the ability of WT CD4+ effector T cells to transfer allergic skin inflammation to Ltb4r1−/− recipients. Pharmacologic blockade of LTB4 synthesis inhibited allergic skin inflammation elicited by cutaneous antigen challenge in previously EC-sensitized mice. Our results demonstrate that a neutrophil-T cell axis reliant on LTB4-BLT1 interaction is required for allergic skin inflammation.
Introduction
Eicosanoids are biologically active lipid mediators that are rapidly generated at sites of injury or inflammation. Leukotrienes (LTs) and prostaglandins are two classes of eicosanoids generated by the metabolism of arachidonic acid through the 5-lipoxygenase (5-LO) pathway and the cyclooxygenase pathway, respectively (Funk, 2001). 5-LO expression and LT generation are generally restricted to myeloid leukocytes, particularly neutrophils, eosinophils, monocytes, macrophages, and mast cells (Werz, 2002). LTs consist of LTB4 and of cysteinyl leukotrienes (cys-LTs), which include LTC4, LTD4, and LTE4. The relative amounts of LTB4 and cys-LTs depend on the relative expression of the 5-LO distal enzymes LTA4 hydrolase (LTA4H) and LTC4 synthase (LTC4S), respectively. LTB4 is generated from innate immune cells such as neutrophils, macrophages, and mast cells in response to a variety of stimuli (Peters-Golden and Henderson, 2007). It primarily acts on myeloid leukocytes causing activation of integrins, adhesion to endothelium walls, and chemotaxis (Goodarzi et al., 2003; Huang et al., 1998; Patcha et al., 2004).
Two receptors for LTB4 have been identified: BLT1 and BLT2. BLT1 is a high-affinity receptor for LTB4 predominantly expressed on neutrophils, macrophages, eosinophils, and activated T cells, with little expression, if any, on resting T cells. BLT2 exhibits low-affinity binding for LTB4 and is ubiquitously expressed (Friedrich et al., 2003). All LTB4 effects on both neutrophils and T cells are lost in Ltb4r1−/− mice (Tager et al., 2003; Tager et al., 2000). LTB4 has been implicated in the pathogenesis of allergic diseases (Ohnishi et al., 2008). Nocturnal concentrations of LTB4 and cys-LTs are elevated in the bronchoalveolar lavage (BAL) fluid of patients with nocturnal asthma (Wenzel et al., 1995). Zileuton, a specific inhibitor of 5-LO, has been shown to decrease LTB4 concentrations in BAL fluid, raising the possibility that its beneficial effect in asthma may involve decreased production of both cys-LTs and LTB4 (Wenzel et al., 1995). Studies on Ltb4r1−/− mice have suggested an important role of the LTB4-BLT1 pathway in the recruitment of effector CD4+ and CD8+ T cells to antigen-challenged airways in a classical mouse model of asthma (Miyahara et al., 2005; Tager et al., 2003; Terawaki et al., 2005).
Atopic dermatitis (AD) is an inflammatory skin disease characterized by itching and scratching, dermal infiltration with Th2 cells and a systemic Th2 cell-mediated response with elevated serum IgE and eosinophilia. Neutrophils from AD patients have elevated LTA4H activity (Okano-Mitani et al., 1996), and release more LTB4 in response to several stimuli (Hilger et al., 1991). LTB4 concentrations are elevated in lesional skin of patients with AD (Fogh et al., 1989; Koro et al., 1999; Ruzicka et al., 1986; Thorsen et al., 1990). However, little is known about the role of LTB4 in AD. To this end, we have developed a mouse model of allergic skin inflammation with many similarities to AD. In this model, repeated epicutaneous (EC) sensitization of tape-stripped skin with ovalbumin (OVA) results in a Th2 cell dominated systemic immune response characterized by dermal infiltration of CD4+ T cells and eosinophils and increased local expression of Th2 cell-derived cytokines (Spergel et al., 1998; Spergel et al., 1999). We have used this model to examine the potential role of LTB4 in allergic skin inflammation. Our findings indicate that LTB4-mediated attraction of neutrophils to skin subjected to tape stripping, a surrogate for scratching, is essential for the development of allergic inflammation at cutaneous sites of antigen introduction.
Results
Scratching and tape stripping increase neutrophils and neutrophil-derived LTB4 and BLT1 in the skin
Mechanical injury to the skin by scratching triggers flares in patients with AD (Fleischer and Boguniewicz, 2010). We examined whether scratching triggers a cellular infiltrate in the skin. There were virtually no detectable neutrophils in the dermis of unscratched skin. In contrast, neutrophils, which were easily differentiated from other inflammatory cells by their characteristic multilobed nucleus, accumulated in the dermis of scratched skin (Fig. 1A,B). There was also an increase of one cell layer in epidermal thickness in scratched skin sites (Fig. 1A). LTB4 is a potent chemoattractant for neutrophils, which express the LTB4 receptor BLT1 (Peters-Golden and Henderson, 2007). Levels of LTB4 and BLT1 mRNA were significantly elevated in scratched skin compared to control unscratched skin (Fig. 1C,D).
Figure 1. Mechanical skin injury results in the accumulation of neutrophils, LTB4 and BLT1 mRNA in human and mouse skin.
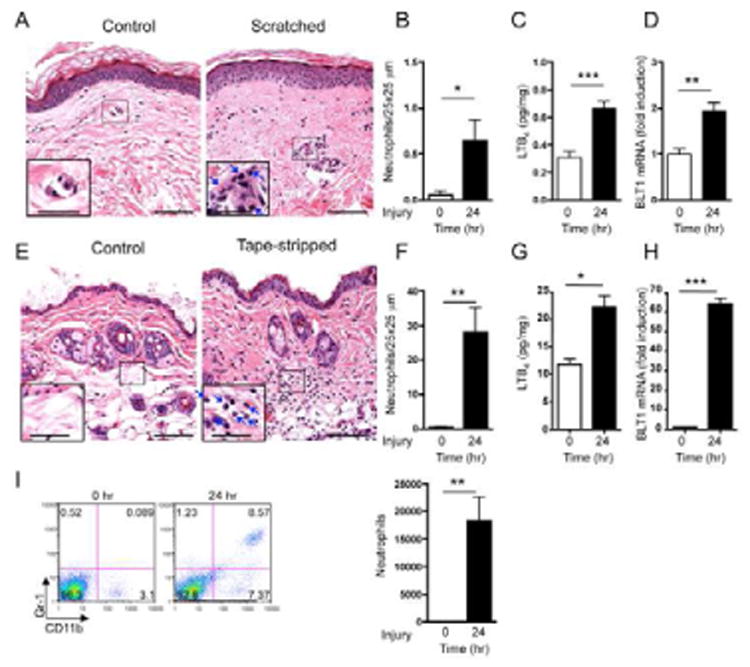
A-D. Representative photomicrographs (A), quantification of neutrophils (B), LTB4 concentrations (pg/mg wet skin tissue) (C), and BLT1 mRNA expression (D) in scratched and unscratched human skin (n=2). Arrows indicate neutrophils. Magnification 200×, inset 400×. Scale bars: 70 μm, inset 35 μm. BLT1 mRNA levels are expressed as fold induction relative to unmanipulated skin. E-H. Representative photomicrographs (E), quantification of neutrophils (F), LTB4 concentrations (pg/mg wet skin tissue) (G), and BLT1 mRNA expression (H) in tape-stripped and unmanipulated back skin of C57BL/6 mice (n=4). I. Representative flow cytometry analysis and quantification of CD11b+Gr-1+ cells in tape-stripped and unmanipulated ear skin of C57BL/6 mice (representative of 4). Columns and error bars represent mean and SEM. *p<0.05, **p<0.01, ***p<0.001.
We examined whether tape stripping, a surrogate for scratching, causes neutrophil accumulation in mouse skin. Shaved dorsal skin of C57BL/6 mice was tape-stripped six times with Tegaderm and examined 24 hrs later. Histologic examination revealed that there were virtually no detectable neutrophils in the dermis of shaved non tape-stripped skin. In contrast, neutrophils were abundant in the dermis of tape-stripped skin (Fig. 1E,F). There was an increase in epidermal and dermal thickness in tape-stripped skin sites compared to non tape-stripped skin (27.24±1.4 mm vs. 8.04±0.4 mm, and 252.9±11.4 mm vs. 154.9±11.9 mm, n=4, p<0.001 each). The amount of LTB4 and BLT1 mRNA were significantly elevated in tape-stripped skin compared to non tape-stripped skin (Fig. 1G,H). An influx of neutrophils was also observed in ear skin 24 hrs after tape stripping six times with Tegaderm, as determined by flow cytometry analysis of cell suspensions from trypsin digested skin for the presence of CD11b+Gr-1+ cells (Fig. 1I).
Neutrophils, but not mast cells, are the source of elevated LTB4 in tape-stripped mouse skin
Neutrophil influx, LTB4 accumulation, and expression of BLT1 mRNA in tape-stripped skin were comparable in mast cell-deficient WBB6F1/J-KitW/KitW-v (KitW/KitW-v) mice and WT WBB6F1 controls (Fig. 2A-C). Neutrophil depletion in C57BL/6 mice by Gr-1 mAb resulted in the loss of >99% of the CD11b+Gr-1+ cells from the blood (Fig. S1A), and strongly impaired the accumulation of neutrophils, LTB4 and BLT1 mRNA in tape-stripped ear skin, compared to treatment with isotype control antibody (Fig. 2D-F). The role of neutrophils in the accumulation of LTB4 and BLT1 mRNA in tape-stripped skin was confirmed in mice depleted of neutrophils by treatment with cyclophosphamide (CTX) (Fig. 2G-I), which resulted in >98% depletion of circulating blood neutrophils (Fig. S1B). These results suggest that the accumulation of LTB4 and BLT1 mRNA in tape-stripped mouse skin is largely dependent on neutrophils.
Figure 2. Neutrophils but not mast cells are the source of increased LTB4 in tape-stripped skin.
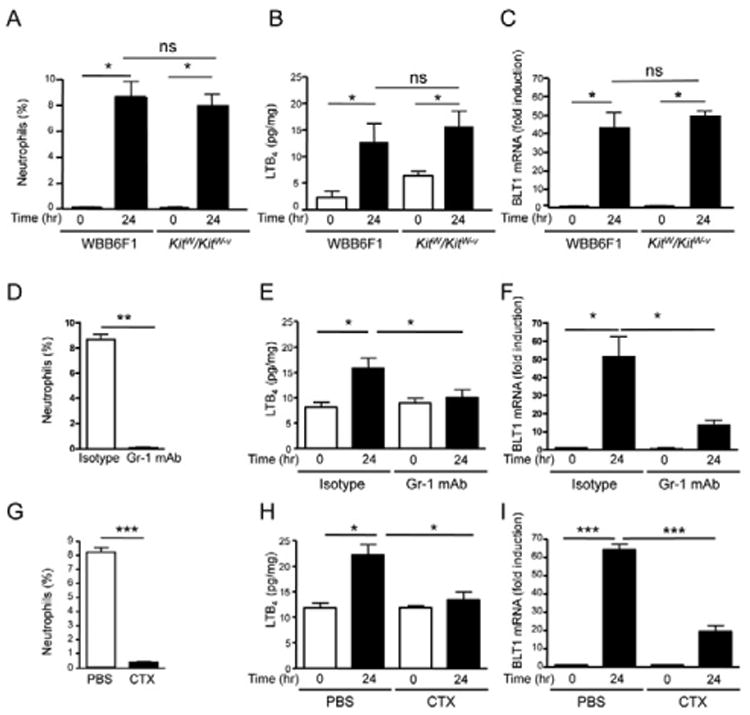
A-I. CD11b+Gr-1+ neutrophils (% of total ear cells), LTB4 concentrations (pg/mg wet skin tissue), and BLT1 mRNA expression in tape-stripped and unmanipulated ear or back skin of KitW/KitW-v mice and WBB6F1 controls (A-C), of tape-stripped C57BL/6 mice treated with Gr-1 mAb or isotype control (D-F), and of tape-stripped C57BL/6 mice treated with CTX or vehicle (G-I). Ear skin was used for A, D, and G. n=3 per group for A-I. BLT1 mRNA levels are expressed as fold induction relative to unmanipulated skin. Columns and error bars represent mean and SEM. *p<0.05, **p<0.01, ***p<0.001. ns= not significant. See also Figure S1.
Neutrophil influx in tape-stripped mouse skin is largely dependent on BLT1 and neutrophil production of LTB4
Kinetic analysis revealed that neutrophil accumulation in tape-stripped ear skin of WT mice, peaked at 24 hrs and waned by 72 hrs (Fig. 3A), as recently reported (Gregorio et al., 2010; Guiducci et al., 2010). There was markedly less accumulation of neutrophils in tape-stripped ear skin of Ltb4r1−/− mice compared to WT controls at all time points (Fig. 3A). The residual accumulation of neutrophils in Ltb4r1−/− mice may be due to robust induction by tape stripping of mRNA for neutrophil chemoattracting chemokines, such as CXCL2, interleukin-1β (IL-1β and IL-6; both the rapid induction (within 6 hrs) of these cytokines and neutrophil influx into tape-stripped skin, were blunted in mice lacking myeloid differentiation primary response gene 88 (Myd88) (Fig. 3B,C). These results indicate that neutrophil influx in tape-stripped mouse skin is largely dependent on BLT1.
Figure 3. Neutrophil accumulation in tape-stripped skin is dependent on their expression of BLT1 and LTA4H.
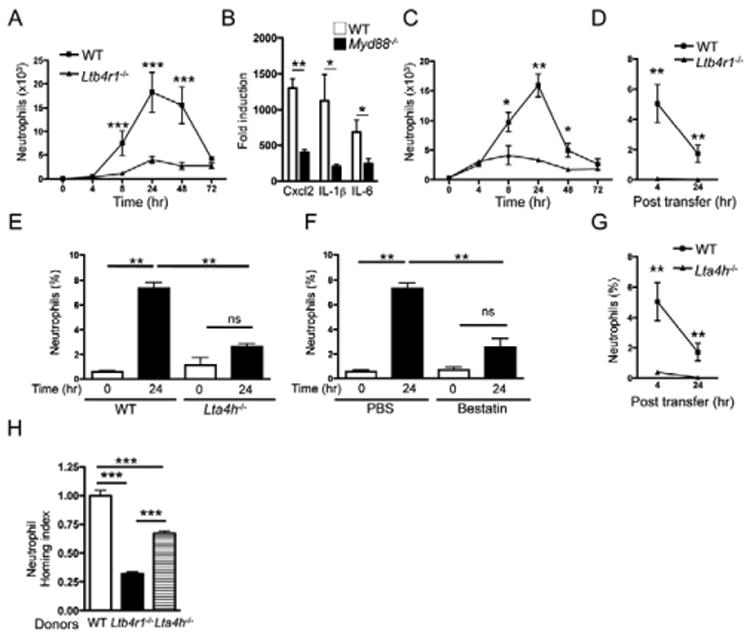
A. Number of neutrophils in WT and Ltb4r1−/− ear skin at different time points (hr) after tape stripping. B. Induction of CXCL2, IL-1β, and IL-6 mRNA expression 6 hrs after tape stripping in the skin of WT and Myd88−/− mice on BALB/c background. mRNA levels are expressed as fold induction relative to unstripped WT skin (0 hrs) (n=4 per group). C. Percentages of neutrophils (% total ear cells) in ear skin of Myd88−/− mice and WT controls at different time points after tape stripping (n=5 per group). D. Percentage of neutrophils (% total ear cells) from WT and Ltb4r1−/− donors in tape-stripped ears of CTX-treated WT recipients (n=4 per group). E, F. Percentage of neutrophils (% total ear cells) in WT and Lta4h−/− ear skin 24 hrs after tape stripping (E), and in WT mice treated with bestatin or vehicle control prior to tape stripping (F). G. Accumulation of neutrophils (% total ear cells) from WT and Lta4h−/− donors in tape-stripped ears of CTX-treated WT recipients (n=4 per group). H. Homing index of Ltb4r1−/− and Lta4h−/− neutrophils in tape-stripped ear skin of CTX-treated WT recipients (n=4 per group). Experiments D and G were performed together and thus share the same WT controls. Columns and error bars represent mean and SEM (n=4 mice per group). *p<0.05, ** p<0.01, *** p<0.001. ns = not significant. See also Figure S2.
To examine whether BLT1 expression by neutrophils is important for their accumulation in tape-stripped skin, we compared the ability of adoptively transferred neutrophils purified from bone marrow of CD45.2+ WT and Ltb4r1−/− mice to accumulate in tape-stripped ears of CD45.1+ WT recipients depleted of neutrophils by CTX. Because of the short half-life of neutrophils, the recipients' ears were tape-stripped 20 hrs before neutrophil transfer to allow for expression of inflammatory cytokines in injured skin. Adoptively transferred WT neutrophils, but not Ltb4r1−/− neutrophils, accumulated in recipient ears 4 hrs, and, to a lesser extent, 24 hrs, post transfer (Fig. 3D). The rise in blood neutrophil count and the percentages of CD45.2+ donor neutrophils in the spleens 4 hrs post transfer were comparable in recipients of WT and Ltb4r1−/− neutrophils (data not shown), indicating that the general circulation of Ltb4r1−/−neutrophils was not impaired.
Neutrophil accumulation in ear skin 24 hrs after tape stripping was severely diminished in Lta4h−/− mice compared to WT controls (Fig. 3E), and in WT mice treated with the LTA4H inhibitor bestatin (Fig. 3F). Since most of the LTB4 that accumulated in tape-stripped skin was derived from neutrophils, we examined whether neutrophil-derived LTB4 was important for the optimal accumulation of neutrophils in tape-stripped skin. There was minimal influx of adoptively transferred CD45.2+ Lta4h−/− neutrophils, which cannot synthesize LTB4, into tape-stripped ears of CTX-treated CD45.1+ WT recipients, compared to CD45.2+ WT neutrophils (Fig. 3G). The percentages of CD45.2+ donor neutrophils in the spleens were comparable in recipients of Lta4h−/− and WT neutrophils (data not shown).
Neutrophil-derived LTB4 may act in an autocrine and/or paracrine manner to promote neutrophil recruitment to tape-stripped skin. To address this issue, we performed co-transfer experiments in which Lta4h−/−, Ltb4r1−/− or WT neutrophils loaded with CMTMR dye (red) were mixed with equal numbers of WT neutrophils loaded with CMFDA dye (green) and adoptively transferred into CTX-treated WT recipients. Four hours later, ear cells were analyzed and the homing index of Lta4h−/− and Ltb4r1−/− neutrophils was calculated relative to that of WT neutrophils, set at 1. Co-administration of WT neutrophils partially, but not completely, restored the influx of Lta4h−/− neutrophils to tape-stripped skin (Fig. 3H), indicating both paracrine and autocrine roles of neutrophil-derived LTB4 in the recruitment of neutrophils to mechanically injured skin. Ltb4r1−/− neutrophils co-transferred with WT neutrophils accumulated significantly less than Lta4h−/− neutrophils co-transferred with WT neutrophils (Fig. 3H), supporting a paracrine component to the action of neutrophil-derived LTB4.
The role of LTB4 in neutrophil recruitment to the skin was not limited to mechanical injury induced by tape stripping. Treatment of mice with bestatin resulted in a significant reduction in neutrophil recruitment to sites of cutaneous infection with S. aureus (Fig. S2A) and in increased bacterial counts and development of larger lesions at these sites (Fig. S2B,C). Bestatin treatment also significantly inhibited the recruitment of neutrophils in response to sterile wounding of the skin (Fig. S2D), and resulted in significantly larger skin lesions on day 1 post-wounding (Fig. S2E).
BLT1 expression by both T cells and non-T cells is essential for allergic skin inflammation
BLT1 expression on T cells has been shown to be important in a mouse model of antigen-driven allergic airway inflammation (Tager et al., 2003). Consistent with this finding, Ltb4r1−/− mice failed to develop allergic skin inflammation following EC sensitization with OVA, as evidenced by their failure to increase dermal infiltration by CD4+ T cells and eosinophils, and upregulate IL-4 and IL-13 mRNA expression in OVA-sensitized skin (Fig. S3A,B). Impaired skin inflammation in Ltb4r1−/− mice was not due to failure to mount a systemic immune response to EC sensitization as their OVA specific antibody concentrations and cytokine secretion by OVA-stimulated splenocytes were comparable to WT controls (Fig. S3C,D).
To investigate the role of BLT1 expression on T and non-T cells in allergic skin inflammation, CD4+ donor T cells from EC-sensitized WT and Ltb4r1−/− mice were examined for their capacity to transfer allergic skin inflammation to WT and Ltb4r1−/− recipients. OVA stimulated CD4+ splenocytes from EC-sensitized mice were adoptively transferred by intravenous (i.v.) injection to naïve recipients, which were challenged with OVA on shaved and tape-stripped back skin on days 0 and 3 after transfer, and examined on day 7. As previously reported in BALB/c mice (He et al., 2008), CD4+ T cells from C57BL/6 WT donors EC-sensitized with OVA transferred allergic skin inflammation to WT recipients, as evidenced by significant increases in epidermal and dermal thickness, number of CD4+ T cells, and IL-4 and IL-13 mRNA expression at sites of OVA challenge compared to sites of saline challenge (Fig. 4 and data not shown). CD4+ T cells from Ltb4r1−/− donors EC-sensitized with OVA failed to transfer allergic skin inflammation to WT recipients. Unexpectedly, CD4+ T cells from WT donors EC-sensitized with OVA failed to transfer allergic skin inflammation to Ltb4r1−/−recipients (Fig. 4A-D), indicating a critical role for BLT1 expression on non-T cells in our model. As expected, CD4+ T cells from Ltb4r1−/− donors EC-sensitized with OVA failed to transfer allergic skin inflammation to Ltb4r1−/− recipients. Recipients of CD4+ T cells from OVA-sensitized donors failed to develop allergic skin inflammation in response to skin challenge with saline (data not shown). Transfer of CD4+ T cells from saline-sensitized mice failed to elicit detectable skin inflammation in OVA-challenged skin of recipients (data not shown).
Figure 4. BLT1 expression by both T cells and non-T cells is essential for allergic skin inflammation.
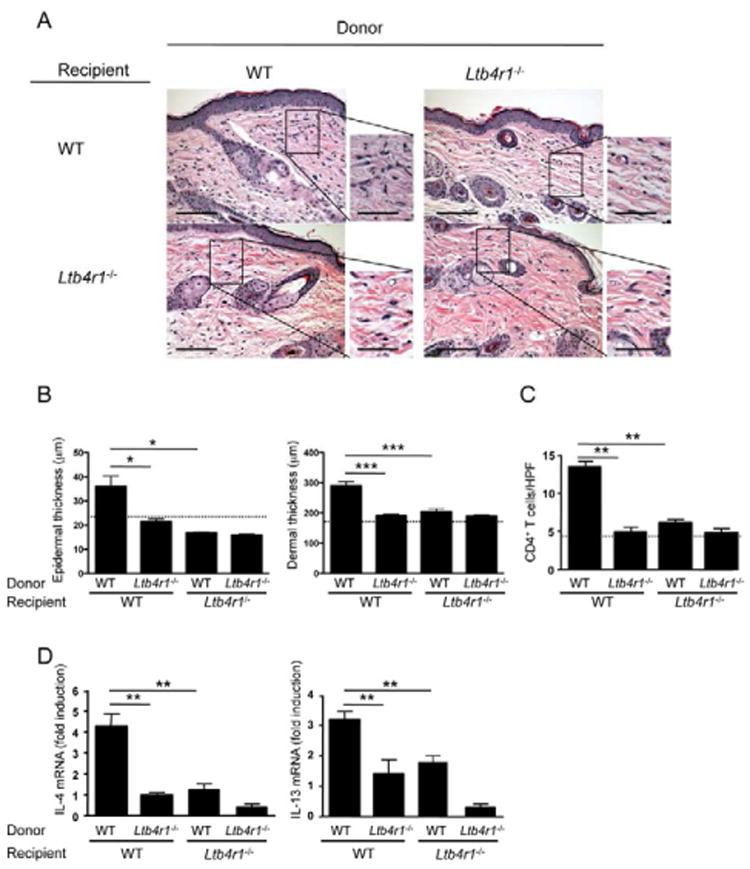
A. Representative photomicrographs of H&E sections from OVA-challenged skin of WT and Ltb4r1−/− recipients of CD4+ T cells from WT or Ltb4r1−/− mice EC-sensitized with OVA. n=5 for each of the 4 groups. Magnification 200×, inset 400×. Scale bars: 100 μm, inset 50 μm. B, C. Epidermal and dermal thickness (B), and numbers of infiltrating CD4+ T cells (C). The dotted line in B and C represents the mean value for OVA-challenged skin of recipients of CD4+ T cells from saline-sensitized WT donors. D. mRNA levels for IL-4 and IL-13 expressed as fold induction relative to OVA-challenged skin of recipients of CD4+ T cells from saline-sensitized WT donors (n=5). Columns and error bars represent mean and SEM. *p<0.05, **p<0.01, *** p<0.001. See also Figure S3.
Expression of BLT1 by neutrophils is important for the development of allergic skin inflammation
To determine whether BLT1 expression on neutrophils is important for the development of allergic skin inflammation, we compared WT and Ltb4r1−/− neutrophils for their capacity to rescue the transfer allergic skin inflammation by OVA-activated CD4+ effector T cells from EC-sensitized WT mice to Ltb4r1−/− recipients. Neutrophils were administered i.v. (107 per mouse) at days 0 and 3 of the one week-long epicutaneous challenge with OVA (Fig. 5A). Administration of WT neutrophils rescued the ability of WT CD4+ T cells to transfer allergic skin inflammation to Ltb4r1−/− recipients. This was evidenced by significant increases in epidermal and dermal thickness, numbers of CD4+ T cells in the dermis and expression of IL-4 and IL-13 mRNA, compared to skin of mice that received only WT CD4+ T cells (Fig. 5A-D). In contrast, administration of Ltb4r1−/− neutrophils failed to rescue the ability of WT CD4+ T cells to transfer allergic skin inflammation to Ltb4r1−/− recipients. These results demonstrate that BLT1 expression by neutrophils is critical for the development of allergic skin inflammation.
Figure 5. WT, but not Ltb4r1−/− neutrophils restores allergic skin inflammation in Ltb4r1−/− recipients.
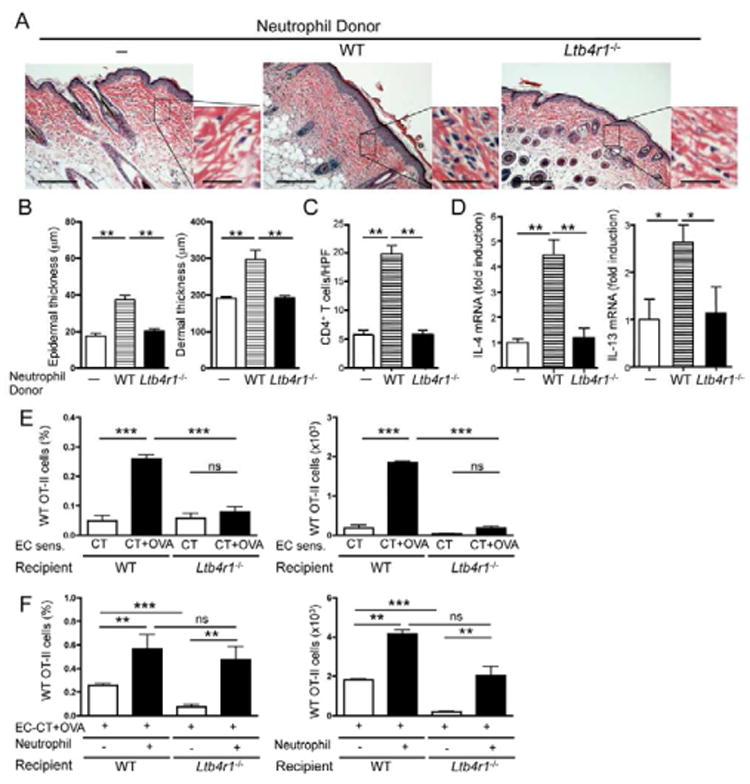
A. Representative photomicrographs of H&E sections from OVA-challenged skin of Ltb4r1−/− recipients of CD4+ T cells from OVA-sensitized WT mice transferred alone (n=3) or with neutrophils from WT (n=5) or Ltb4r1−/− donors (n=4). Magnification 100×, inset 400×. Scale bars: 200 mm, inset 50 mm. B-D. Epidermal and dermal thickness (B), number of infiltrating CD4+ T cells (C), and mRNA levels of IL-4 and IL-13 expressed as fold induction relative to recipients of CD4+ T cells from OVA-sensitized WT mice transferred without neutrophils (D). E. Percentage (% total ear cells) and number of Vα2+Vβ5+ transgenic OT-II CD4+ T cells from WT donors accumulating in OVA-challenged ear skin of WT and Ltb4r1−/− recipients. F. Effect of co-administration of WT neutrophils on the percentage (% total ear cells) and number of Vα2+Vβ5+ transgenic OT-II CD4+ T cells from WT donors which accumulate in OVA-challenged ear skin of WT and Ltb4r1−/− recipients. Columns and error bars represent mean and SEM (n=3-5 mice per group). *p<0.05, **p<0.01, *** p<0.001. ns = not significant. See also Figure S4.
Accumulation of effector T cells to antigen-challenged skin requires BLT1 expression by neutrophils
Failure of Ltb4r1−/− neutrophils to rescue the ability of WT CD4+ T cells to transfer allergic skin inflammation to Ltb4r1−/− recipients prompted us to directly investigate the role of BLT1 expression by neutrophils in the accumulation of antigen specific effector CD4+ T cells into cutaneous sites of antigen challenge. We examined the ability of CD4+ OT-II T cells, activated with OVA peptide and antigen presenting cells (APCs) for 4 days in vitro, to accumulate into the tape-stripped ear skin of recipients following challenge with OVA+cholera toxin (CT) or CT alone, as control. Vα2+Vβ5+ WT OT-II effector T cells accumulated significantly more in antigen-challenged skin than in control challenged ear skin of WT recipients (Fig. 5E). In contrast, WT OT-II effector T cells failed to accumulate in antigen-challenged skin of Ltb4r1−/− recipients (Fig. 5E), indicating that BLT1 expression by non-T cells is important for the homing of CD4+ effector T cells to the skin. Ltb4r1−/− OT-II effector T cells failed to accumulate in antigen-challenged skin of WT recipients (Fig. S4A), despite normal proliferation to OVA (data not shown) and normal expression of the skin homing receptor CCR4 and memory marker CD44 (Fig. S4B,C), confirming the role of BLT1 expression on T cells in homing to the skin.
Co-administration of WT neutrophils i.v. significantly increased the accumulation of adoptively transferred WT OT-II effector T cells into antigen-challenged skin of WT recipients (Fig. 5F). More importantly, it completely rescued the ability of WT OT-II effector T cells to accumulate in antigen-challenged skin of Ltb4r1−/− recipients (Fig. 5F). These results demonstrate that BLT1 expression by neutrophils is critical for the accumulation of CD4+ effector T cells into cutaneous sites of antigen challenge and for allergic skin inflammation.
Neutrophil-derived LTB4 is important for the development of allergic skin inflammation
We next investigated whether LTB4 production by skin-infiltrating neutrophils important for the development of allergic skin inflammation. To this purpose, we examined whether Lta4h−/− neutrophils rescued the ability of OVA-activated CD4+ effector T cells from EC-sensitized WT mice to transfer allergic skin inflammation to Ltb4r1−/− recipients. Because Lta4h−/− neutrophils failed to migrate to tape-stripped skin, neutrophils from Lta4h−/− mice and WT controls were injected intradermally (i.d.) on days 0 and 3 at the site of EC challenge. Intradermal administration of WT neutrophils rescued the ability of WT CD4+ T cells to transfer allergic skin inflammation to Ltb4r1−/− recipients, evidenced by significant increases in epidermal and dermal thickness, CD4+ T cell infiltration in the dermis, and expression of IL-4 and IL-13 mRNA, compared to antigen-challenged skin of mice that received only WT CD4+ T cells (Fig. 6A-D). In contrast, i.d. injection Lta4h−/− neutrophils in Ltb4r1−/− recipients failed to rescue the ability of WT CD4+ T cells to transfer allergic skin inflammation to Ltb4r1−/− recipients. In addition, i.d. administration of Ltb4r1−/− neutrophils, which have an intact ability to produce LTB4, caused an increase in the accumulation of OT-II cells in EC challenged ear skin of Ltb4r1−/− recipients, and in ear scratching, comparable to those caused by i.d. administration of WT neutrophils (Fig. 6E,F).
Figure 6. Intradermal injection of Lta4h−/− neutrophils fails to restore allergic skin inflammation in Ltb4r1−/− recipients.
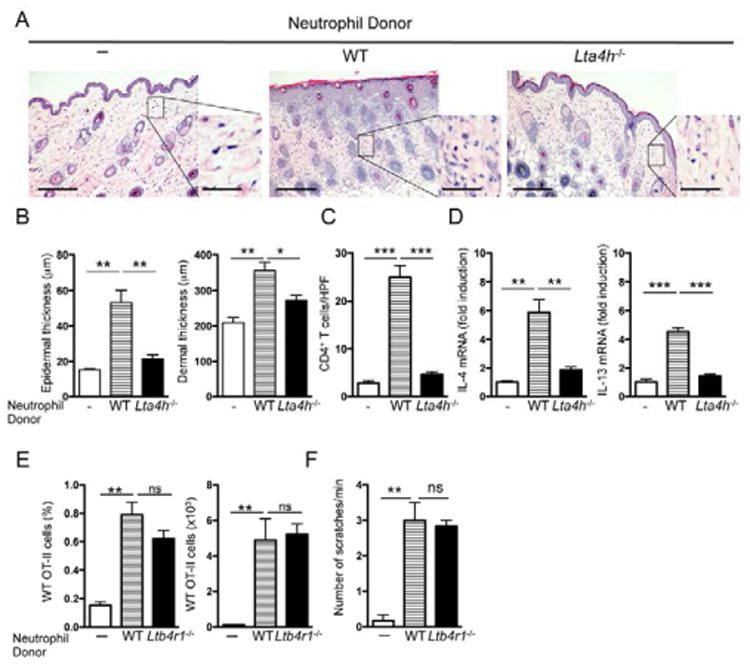
A. Representative photomicrographs of H&E sections from OVA-challenged skin of Ltb4r1−/− recipients of CD4+ T cells from OVA-sensitized WT mice transferred alone or with neutrophils from WT or Lta4h−/− donors (n=3 per group). Magnification 100×, inset 400×. Scale bars: 200 μm, inset 50 μm. B-D. Epidermal and dermal thickness (B), number of infiltrating CD4+ T cells (C), and mRNA levels of IL-4 and IL-13 expressed as fold induction relative to recipients of CD4+ T cells from OVA-sensitized WT mice transferred without neutrophils (D). E, F. Effect of i.d. injection of WT and Ltb4r1−/− neutrophils on the homing of WT OT-II cells to OVA challenged skin of Ltb4r1−/− recipients (E) and on ear scratching by the recipients (F). Columns and error bars represent mean and SEM. n=3-4 mice per group. *p<0.05, **p<0.01, ***p<0.001. See also Figure S5.
LTB4 induces itch-associated scratching response in mice (Kim et al., 2007). Tape stripping induced significantly more scratching in WT mice than in Ltb4r1−/− and Lta4h−/− mice, suggesting that LTB4 is important in the scratching response to mechanical injury (Fig. S5A). EC-sensitized with OVA exhibited increased scratching (Fig. S5B), and LTB4 concentrations were elevated in OVA sensitized mouse skin sites (Fig. S5C), as in AD skin lesions. These results suggest that generation of LTB4 by infiltrating neutrophils is critical for their ability to promote T cell mediated allergic skin inflammation.
Pharmacologic blockade of LTB4 synthesis inhibits acute skin flares in previously EC-sensitized mice
Cutaneous exposure to antigens provokes skin flares in AD patients (Nosbaum et al., 2010; Sicherer and Leung, 2007). Therefore, we next investigated whether administration of bestatin to EC-sensitized mice prevented the development of allergic skin inflammation following cutaneous antigen challenge. C57BL/6 mice were EC-sensitized with OVA for 7 weeks then immediately challenged by application of OVA to a previously unsensitized shaved and tape-stripped skin site on the back. Mice were treated by intraperitoneal (i.p.) injection with bestatin (0.4 mg/day) or vehicle control, daily for 7 days beginning 2 days before the seven-day challenge (Fig. 7A). Mice treated with vehicle alone developed allergic skin inflammation in response to cutaneous OVA challenge, as evidenced by significantly increased epidermal and dermal thickness, dermal infiltration with CD4+ T cells, and expression of Th2 cell-derived cytokines, compared to challenge with saline (Fig. 7B-E). In contrast, mice treated with bestatin failed to develop allergic skin inflammation in response to cutaneous OVA challenge. These results suggest that inhibition of LTB4 production prevents allergic skin inflammation driven by cutaneous antigen exposure in previously sensitized mice.
Figure 7. Pharmacologic blockade of LTB4 synthesis inhibits acute skin flares in previously EC-sensitized mice.
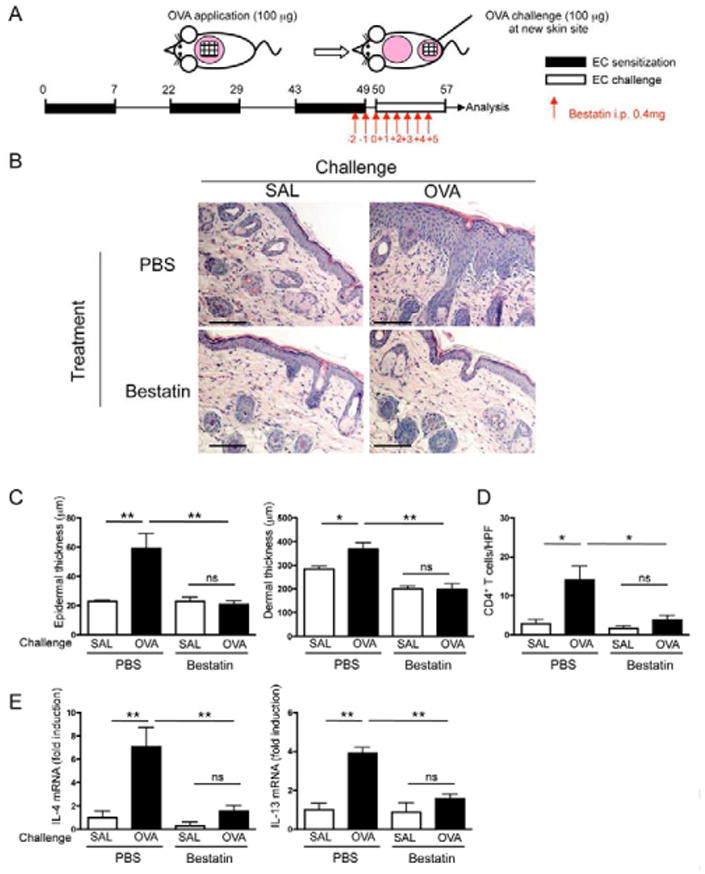
A. Experimental protocol. B. Representative photomicrographs of H&E stained sections from challenged skin of OVA-sensitized mice treated with bestatin or vehicle (PBS) control. Magnification 200×. Scale bars: 100 μm. C-E. Epidermal and dermal thickness (C), number of infiltrating CD4+ T cells (D), and mRNA levels of IL-4 and IL-13 expressed as fold induction relative to saline challenged untreated mice (E). Columns and error bars represent mean and SEM. n=3 mice per group. *p<0.05, **p<0.01. ns = not significant.
Discussion
In this study, we have identified a critical role for neutrophils in allergic skin inflammation in a mouse model with features of AD. We demonstrated that LTB4 production by neutrophils was essential for their accumulation in mechanically injured skin and for the recruitment of effector CD4+ T cells to cutaneous sites of antigen exposure and development of allergic skin inflammation.
Mechanical skin injury by scratching in humans, and its surrogate, tape stripping in mice, caused robust infiltration of neutrophils and accumulation of LTB4 and mRNA for its receptor, BLT1, in the dermis. The slight increase in epidermal thickness in injured human and mouse skin is possibly due to the release of cytokines that promote keratinocyte proliferation, which include IL-6 (Werner et al., 2007). Accumulation of neutrophils and BLT1 mRNA was substantially greater in tape-stripped mouse skin than in scratched human skin, possibly because mouse skin is much thinner than human skin and might be more susceptible to mechanical skin injury. The relatively comparable elevation of LTB4 concentrations in tape-stripped mouse skin and scratched human skin suggests a more rapid washout of LTB4 from injured mouse skin. The baseline concentration of LTB4 may reflect a background signal intrinsic to the assay used and/or be derived from resident cells in the skin and may have not been biologically active, since neutrophils were not detected in unmanipulated skin. Studies in Lta4h−/− and Ltb4r1−/− mice demonstrated that LTB4 and BLT1 are both important for neutrophil accumulation into tape-stripped mouse skin. The role of LTB4 in neutrophil recruitment to the skin is supported by the observation that epicutaneous application of LTB4 in normal healthy volunteers results in local neutrophil infiltration (de Jong et al., 1992). LTB4 induces itch-associated scratching response in mice (Kim et al., 2007). Tape stripping induced significantly more scratching in WT mice than in Ltb4r1−/− and Lta4h−/− mice, suggesting that LTB4 is important in the scratching response to mechanical injury.
Neutrophils, but not mast cells, were the major source of the increased LTB4 in mouse skin 24 hrs following mechanical injury. LTB4 failed to accumulate in mice depleted of neutrophils using Gr-1 mAb or CTX, but accumulated normally in the mechanically injured skin of mast cell-deficient KitW/KitW-v mice. Although mast cells could release LTB4 early after mechanical injury, mast cell-derived LTB4 was clearly not essential for neutrophil influx into mechanically injured skin. This is consistent with our previous observation that allergic skin inflammation elicited by EC sensitization of tape-stripped skin with OVA is intact in mast cell-deficient KitW/KitW-v mice (Alenius et al., 2002).
Adoptive transfer experiments demonstrated that neutrophil influx in tape-stripped mouse skin was largely dependent on BLT1 and on both paracrine and autocrine actions of neutrophil-derived LTB4. LTB4 played a role in neutrophil recruitment to the skin in response to bacterial infection and sterile wounding. In addition, neutrophil-derived LTB4 is required for the recruitment of neutrophils to the joints and for the development of inflammatory arthritis (Chen et al., 2006; Chou et al., 2010; Kim et al., 2006). These observations suggest that LTB4 generated by neutrophils is important for their recruitment to the skin in response to a variety of inflammatory stimuli, as well as for their recruitment to other tissues.
Additional factors, which include inflammatory chemokines and cytokines, produced by resident skin cells may contribute to the initial neutrophil influx in the tape-stripped skin, as suggested by the residual neutrophil infiltrate observed of Ltb4r1−/− mice. Furthermore, production of IL-1b and IL-6 by skin infiltrating WT neutrophils may explain the finding that Ltb4r1−/− neutrophils could be detected in tape-stripped skin when co-transferred with WT neutrophils, but not when transferred alone. Both the rapid induction (within 6 hrs) of CXCL2, IL-1b and IL-6 mRNA and neutrophil influx into tape-stripped skin, were blunted in Myd88−/− mice. This observation suggests that signaling via TLR receptors, possibly by endogenous alarmins (Guiducci et al., 2010) and/or signaling via IL-1 receptor (IL-1R) family members released from damaged keratinocytes, plays an important role in the initial neutrophil influx into injured skin.
A central finding of the present study is that CD4+ T effector cells from OVA-sensitized WT mice fail to transfer allergic inflammation to Ltb4r1−/− recipients. Taken together with the observation that Ltb4r1−/− CD4+ T cells fail to transfer allergic inflammation, this result indicates that the expression of BLT1 on both non-T cells and T cells is important for allergic skin inflammation. Co-transfer of WT neutrophils, but not Ltb4r1−/− neutrophils, rescued the ability of CD4+ effector T cells to accumulate in antigen-challenged skin of Ltb4r1−/− recipients and cause allergic skin inflammation. Intradermal administration of WT, and Ltb4r1−/−, but not Lta4h−/−, neutrophils rescued the ability of WT CD4+ effector T cells to transfer allergic skin inflammation to Ltb4r1−/− recipients. This finding demonstrates that neutrophil-derived LTB4 is critical for the ability of CD4+ effector T cells to cause allergic skin inflammation. These observations demonstrate that LTB4 production by neutrophils is critical both for the BLT1 dependent accumulation of neutrophils and the BLT1 dependent recruitment of antigen specific effector CD4+ T cells to cutaneous sits of antigen introduction.
The LTB4 synthesis inhibitor bestatin blocked neutrophil influx into tape-stripped skin and inhibited the development of allergic inflammation at sites of cutaneous antigen challenge in mice. This result suggests that blockade of LTB4-BLT1 interaction may be a useful strategy in preventing acute flares that are precipitated in quiescent skin in patients with AD by scratching and introduction of previously encountered antigen.
LTB4 concentrations were elevated in OVA sensitized mouse skin sites, as in AD skin lesions, and mice EC-sensitized with OVA exhibited increased scratching. However, unlike AD skin lesions, which contain few, if any, neutrophils (Leiferman, 1994), EC-sensitized mouse skin sites exhibit neutrophil infiltration (Spergel et al., 1998). Neutrophils, like eosinophils, may not persist intact in chronic AD lesions. It thus remains possible that scratching established skin lesions induces a transient acute neutrophil influx, which triggers LTB4-dependent recruitment of BLT1+ effector T cells, resulting in the exacerbation of allergic skin inflammation.
Scratching is a hallmark of AD and causes further disruption of an already abnormal skin barrier, allowing cutaneous introduction of antigen. Our finding that the LTB4-BLT1 axis is implicated in two sequential steps in allergic inflammation at sites of cutaneous antigen exposure, namely recruitment of neutrophils and neutrophil-dependent recruitment of effector T cells, make the LTB4-BLT1 axis a potential therapeutic target in AD.
Experimental Procedures
Human skin biopsy
Two healthy non-allergic adult subjects were asked, after obtaining informed consent, to scratch the inner side of their forearm, for a total of 12 times over a period of 24 hrs (every hour 8 times on day 0, and 4 times on day 1) using each time twenty strokes of their index fingernail. A 4 mm punch biopsy was obtained an hour after the last scratching from the scratched site and from the corresponding unscratched site of the contralateral forearm. Histologic examination was performed on H&E stained sections by an experienced dermatopathologist who was blinded to the source of the material.
Mice and Sensitization
Ltb4r1−/− and Ltb4r1−/− OT-II mice on C57BL/6 background, were previously described (Tager et al., 2003). Lta4h−/− mice, a kind gift of Dr. Koller (Byrum et al., 1999) were bred on C57BL/6 background for 10 generations. WT C57BL/6 mice were obtained from Charles River Laboratory. J-KitW/KitW-v (WBB6F1) mice and congenic WBB6F1 WT mice, OT-II, and CD45.1+ mice on C57BL/6 background were obtained from the Jackson Laboratory. LysEGFP reporter mice on a C57BL/6 genetic background were previously described (Faust et al., 2000).
The animals were kept in a specific pathogen-free environment. All procedures performed on the animals were in accordance with the Animal Care and Use Committee of the Boston Children's Hospital or UCLA Chancellor's Animal Research Committee.
To cause mechanical injury, the shaved back skin or the ear skin were tape-stripped 6 times with Tegaderm (Westnet Inc, Canton, MA). For neutrophil depletion, WT mice were treated with CTX or PBS control as described previously (Zuluaga et al., 2006). In selected experiments, WT mice were treated by i.p. injection with 100 μg Gr-1 mAb (RB6-8C5, ebioscience) or isotype control at day -3 and -1 of tape stripping. Neutrophil depletion was confirmed by flow cytometry at day 0 before tape stripping. Mice were treated by i.p. injection with 0.4 mg/day bestatin (Cayman Chemical) or vehicle control, daily for 2 days before tape stripping.
EC sensitization of six- to eight-week-old female mice was performed as described previously (Spergel et al., 1998). Briefly, the skin of anesthetized mice was shaved and tape-stripped 6 times by transparent IV dressing (Tegaderm®; Owens & Minor Inc., Franklin, Massachusetts, USA). One hundred micrograms of chicken egg ovalbumin (OVA Grade V; Sigma-Aldrich, St. Louis, Missouri, USA) in 100 μL of normal saline, or placebo (100 μL of normal saline), were placed on a patch of sterile gauze (1 × 1 cm), which was secured to the skin with a transparent bio-occlusive dressing. Each mouse had a total of three one-week exposures to the patch separated from each other by two-week intervals. In the acute skin challenge model, mice were EC-sensitized with OVA for 7 weeks then challenged on days 0 and 3 after sensitization (days 50 and 53 from the start) by means of application of OVA or saline to shaved and tape-stripped, previously unsensitized skin and were studied on day 7 of challenge (day 57 from the start). Mice were treated by i.p. injection with 0.4 mg/day bestatin or vehicle control, daily for 7 days beginning 2 days before the seven-day challenge as shown in Fig. 7A.
Antibodies and flow cytometry analysis of ear skin
Ear skin was used for flow cytometry analysis due to a technical difficulty of preparing cell suspension from the dorsal skin. Cells from ears were isolated as described previously (He et al., 2008). Briefly, ears were separated in two halves and floated on trypsin-EDTA (Cellgro) for 30 min at 37°C to allow dissociation of the epidermis. Both epidermal and dermal sheets were incubated for a further 1-2 hr at 37°C. The resulting cell suspensions were stained with the appropriate antibodies and analyzed by flow cytometry.
For surface staining, the following antibodies were used: fluorochrome-labeled anti-CD11b, Gr-1, CD4, CD44, Vα2, Vβ5, CD45.1 and CD45.2 (all from eBioscience). Cells were analyzed on FACSCanto and the data was analyzed using Flowjo software.
Measurement of skin LTB4 concentrations
LTB4 concentrations in human and mouse skin were determined using solid phase extraction followed by EIA kit measurement according to the manufacturer's instructions (Cayman Chemical).
Neutrophil preparation from bone marrow
Mouse neutrophils were prepared from the femur and tibia of WT and Ltb4r1−/− mice using discontinuous Percoll density centrifugation as previously described (Jia et al., 2007). Preparations were >90% pure as determined by flow cytometry analysis with double staining using Gr-1 and CD11b mAbs.
Adoptive transfer of neutrophils
CD45.1+ WT recipient mice were treated with CTX as described above and ear skin were tape-stripped 20 hrs before neutrophil transfer. 1 × 107 bone marrow neutrophils were isolated from CD45.2+ WT, Ltb4r1−/−, and Lta4h−/− mice and i.v. injected into CTX-treated WT mice. The percentage of adoptively transferred neutrophils recruited to the ear skin was analyzed 4 hrs and 24 hrs after transfer using a FACSCanto (BD Biosciences).
In co-transfer experiment, 5 × 106 neutrophils from WT and Ltb4r1−/− mice were labeled with 1 μM CellTracker Green CMFDA (5-chloromethylfluorescein diacetate) and 15 μM CellTracker Orange CMTMR (5-(and-6)-(((4-chloromethyl) benzoyl) amino) tetramethylrhodamine (Invitrogen) at 37°C for 10 minutes. Ltb4r1−/− or WT neutrophils loaded with CMTMR dye (red) were mixed with 5 × 106 WT neutrophils loaded with CMFDA dye (green) at an input ratio of ∼1:1 and adoptively transferred by i.v. into CTX-treated WT recipients whose ears were tape-stripped 20 hrs earlier. Four hours later, the ratio of green:red fluorescent cells in the ears was determined, adjusted to the input ratio and the relative skin homing index was calculated.
Adoptive transfer of allergic skin inflammation
Splenocytes from EC-sensitized WT or Ltb4r1−/− mice were cultured with OVA for 5 days, then CD4+ T cells were purified by AutoMACS and injected i.v. into naïve recipients (5 × 106 CD4+ T cells per mouse) which were challenged the same day by application of 100 mg OVA to shaved and tape-stripped dorsal skin. Skin challenge was repeated on day 3 and the skin site was examined on day 7. In selected experiments, 5 × 106 splenic CD4+ T cells from WT mice EC-sensitized with OVA were injected i.v. into naïve Ltb4r1−/− recipients on day 0 and WT, Ltb4r1−/−, or Lta4h−/−neutrophils were administered i.v. (1 × 107) or i.d. (1 × 106) on day 0 and day 3 of one-week EC challenge.
Skin homing of CD4+ T cells
CD4+ T cells were prepared from the spleen of OT-II mice on WT or Ltb4r1−/− background and activated with irradiated syngeneic splenocytes (3000 rads) (1:10 ratio) as antigen-presenting cells (APCs) in the presence of OVA (100 mg/ml) and IL-2 (10 ng/ml) for 5 days. 5 × 106 activated transgenic CD4+ T cells from WT or Ltb4r1−/− background were injected i.v. into naïve recipients followed by application of OVA+cholera toxin (CT) or CT alone to tape-stripped ear skin as previously described (He et al., 2008). In selected experiments, recipients received WT or Ltb4r1−/− neutrophils i.v. (1 × 107) or i.d. (1 × 106) on day 0 and again on day 3 of one-week EC challenge. At the end of one-week EC challenge, single cell suspension was prepared from ear skin as described above, stained with the appropriate antibodies, and analyzed by flow cytometry.
Quantitative PCR analysis of cytokines and histological analysis
Total RNA was extracted from homogenized skin tissue or from cultured cells with RNAqueous extraction kit (Ambion Inc) following the manufacturer's instructions. cDNA was generated with iScript cDNA synthesis kit (Bio-rad Laboratory). Quantitative real-time PCR was done using Taqman Gene Expression Assay, universal PCR master mix and the ABI Prism 7300 sequence detection system with commercial primers and probes, all from Applied Biosystems. Fold induction of target gene expression was calculated using the comparative method for relative quantitation by normalization to the internal control β2-microglobulin, as described previously (He et al., 2007). For histological analysis, skin specimens were fixed in 10% buffered formalin and embedded in paraffin. Multiple 4 mm sections of skin were stained with hematoxylin and eosin (H&E). Neutrophils were counted in ten randomly chosen areas 25 × 25 μm in size. CD4 staining of skin sections was performed as previously described (Spergel et al., 1998). Epidermal and dermal thickening, eosinophils, and CD4+ T cells were counted blinded in 10-15 high-power fields (HPFs) at a magnification of 400×.
Immunoglobulin determinations
Mice were bled, and serum concentrations of OVA-specific IgE, IgG1, and IgG2a Abs were measured using modified OVA-specific ELISAs as previously described (He et al., 2008).
Response of splenocytes to OVA
Single-cell suspensions from spleen of EC-sensitized mice were cultured in complete RPMI 1640 (Invitrogen) supplemented by 10% FCS, 0.05 mM 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin at 3 × 106/ml in 24-well following the stimulation with OVA (100 μg/ml) for 4 days. Supernatants were collected for cytokine measurements by ELISA.
Statistical analysis
Two-tailed Student's t-test or one-way ANOVA was used to determine statistical differences between groups. A p value smaller than 0.05 was considered statistically significant.
Supplementary Material
Highlights.
Scratching of human and mouse skin causes LTB4-BLT1 dependent neutrophil influx
BLT1 expression on T and non-T cells is required for allergic skin inflammation
Transfer of allergic skin inflammation requires neutrophil-derived LTB4 and BLT1
Pharmacologic blockade of LTB4 synthesis inhibits allergic skin inflammation
Acknowledgments
This work was supported by the Atopic Dermatitis Research Network NIH/NIAID contract HHSN272201000020C (RSG), USPHS grants AR-047417 (RSG), AI 065858-03 (DML) HL-085100, AI-076471, HL-092020, GM-076084 and a Research Scholar Grant from American Cancer Society (HRL) and AI-050892 and AI-040618 (ADL), R01 AI078910 (LSM), T32 AR058921 (JSC), R24 CA92865 (UCLA Small Animal Imaging Resource Program), and the Children's Hospital Boston Faculty Career Development Fellowship (MKO). David M Lee is currently an employee of Novartis Pharma AG and owns stock/options >$10,000, and has ownership and consulting income >$10,000 from Synovex Corp. Mei Chen is currently an employee of Pfizer Inc. We thank Drs. Michel J. Massaad and Hans C. Oettgen for reading the manuscript. We also thank Ms. Jacqueline Beaupre for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alenius H, Laouini D, Woodward A, Mizoguchi E, Bhan AK, Castigli E, Oettgen HC, Geha RS. Mast cells regulate IFN-gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. J Allergy Clin Immunol. 2002;109:106–113. doi: 10.1067/mai.2002.120553. [DOI] [PubMed] [Google Scholar]
- Byrum RS, Goulet JL, Snouwaert JN, Griffiths RJ, Koller BH. Determination of the contribution of cysteinyl leukotrienes and leukotriene B4 in acute inflammatory responses using 5-lipoxygenase- and leukotriene A4 hydrolase-deficient mice. J Immunol. 1999;163:6810–6819. [PubMed] [Google Scholar]
- Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong EM, van Erp PE, van Vlijmen IM, van de Kerkhof PC. The inter-relation between inflammation and epidermal proliferation in normal skin following epicutaneous application of leukotriene-B4--an immunohistochemical study. Clin Exp Dermatol. 1992;17:413–420. doi: 10.1111/j.1365-2230.1992.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–726. [PubMed] [Google Scholar]
- Fleischer AB, Jr, Boguniewicz M. An approach to pruritus in atopic dermatitis: a critical systematic review of the tacrolimus ointment literature. J Drugs Dermatol. 2010;9:488–498. [PubMed] [Google Scholar]
- Fogh K, Herlin T, Kragballe K. Eicosanoids in skin of patients with atopic dermatitis: prostaglandin E2 and leukotriene B4 are present in biologically active concentrations. J Allergy Clin Immunol. 1989;83:450–455. doi: 10.1016/0091-6749(89)90132-2. [DOI] [PubMed] [Google Scholar]
- Friedrich EB, Tager AM, Liu E, Pettersson A, Owman C, Munn L, Luster AD, Gerszten RE. Mechanisms of leukotriene B4--triggered monocyte adhesion. Arterioscler Thromb Vasc Biol. 2003;23:1761–1767. doi: 10.1161/01.ATV.0000092941.77774.3C. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, Arai N, Gallo RL, Digiovanni J, Gilliet M. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010 doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010 doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci U S A. 2007;104:15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger RA, Neuber K, Konig W. Conversion of leukotriene A4 by neutrophils and platelets from patients with atopic dermatitis. Immunology. 1991;74:689–695. [PMC free article] [PubMed] [Google Scholar]
- Huang WW, Garcia-Zepeda EA, Sauty A, Oettgen HC, Rothenberg ME, Luster AD. Molecular and biological characterization of the murine leukotriene B4 receptor expressed on eosinophils. J Exp Med. 1998;188:1063–1074. doi: 10.1084/jem.188.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Subramanian KK, Erneux C, Pouillon V, Hattori H, Jo H, You J, Zhu D, Schurmans S, Luo HR. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5- trisphosphate signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Kim HJ, Sung KS, Kim H, Cho SA, Kim KM, Lee CH, Kim JJ. 12(S)-HPETE induces itch-associated scratchings in mice. European journal of pharmacology. 2007;554:30–33. doi: 10.1016/j.ejphar.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koro O, Furutani K, Hide M, Yamada S, Yamamoto S. Chemical mediators in atopic dermatitis: involvement of leukotriene B4 released by a type I allergic reaction in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 1999;103:663–670. doi: 10.1016/s0091-6749(99)70240-x. [DOI] [PubMed] [Google Scholar]
- Leiferman KM. Eosinophils in atopic dermatitis. The Journal of allergy and clinical immunology. 1994;94:1310–1317. doi: 10.1016/0091-6749(94)90347-6. [DOI] [PubMed] [Google Scholar]
- Miyahara N, Takeda K, Miyahara S, Taube C, Joetham A, Koya T, Matsubara S, Dakhama A, Tager AM, Luster AD, Gelfand EW. Leukotriene B4 receptor-1 is essential for allergen-mediated recruitment of CD8+ T cells and airway hyperresponsiveness. J Immunol. 2005;174:4979–4984. doi: 10.4049/jimmunol.174.8.4979. [DOI] [PubMed] [Google Scholar]
- Nosbaum A, Hennino A, Berard F, Nicolas JF. Patch testing in atopic dermatitis patients. Eur J Dermatol. 2010;20:563–566. doi: 10.1684/ejd.2010.1014. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Miyahara N, Gelfand EW. The role of leukotriene B(4) in allergic diseases. Allergol Int. 2008;57:291–298. doi: 10.2332/allergolint.08-RAI-0019. [DOI] [PubMed] [Google Scholar]
- Okano-Mitani H, Ikai K, Imamura S. Leukotriene A4 hydrolase in peripheral leukocytes of patients with atopic dermatitis. Arch Dermatol Res. 1996;288:168–172. doi: 10.1007/BF02505219. [DOI] [PubMed] [Google Scholar]
- Patcha V, Wigren J, Winberg ME, Rasmusson B, Li J, Sarndahl E. Differential inside-out activation of beta2-integrins by leukotriene B4 and fMLP in human neutrophils. Exp Cell Res. 2004;300:308–319. doi: 10.1016/j.yexcr.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- Ruzicka T, Simmet T, Peskar BA, Ring J. Skin levels of arachidonic acid-derived inflammatory mediators and histamine in atopic dermatitis and psoriasis. J Invest Dermatol. 1986;86:105–108. doi: 10.1111/1523-1747.ep12284061. [DOI] [PubMed] [Google Scholar]
- Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects. The Journal of allergy and clinical immunology. 2007;119:1462–1469. doi: 10.1016/j.jaci.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- Tager AM, Dufour JH, Goodarzi K, Bercury SD, von Andrian UH, Luster AD. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki K, Yokomizo T, Nagase T, Toda A, Taniguchi M, Hashizume K, Yagi T, Shimizu T. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol. 2005;175:4217–4225. doi: 10.4049/jimmunol.175.7.4217. [DOI] [PubMed] [Google Scholar]
- Thorsen S, Fogh K, Broby-Johansen U, Sondergaard J. Leukotriene B4 in atopic dermatitis: increased skin levels and altered sensitivity of peripheral blood T-cells. Allergy. 1990;45:457–463. doi: 10.1111/j.1398-9995.1990.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Wenzel SE, Trudeau JB, Kaminsky DA, Cohn J, Martin RJ, Westcott JY. Effect of 5-lipoxygenase inhibition on bronchoconstriction and airway inflammation in nocturnal asthma. Am J Respir Crit Care Med. 1995;152:897–905. doi: 10.1164/ajrccm.152.3.7663802. [DOI] [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- Werz O. 5-lipoxygenase: cellular biology and molecular pharmacology. Curr Drug Targets Inflamm Allergy. 2002;1:23–44. doi: 10.2174/1568010023344959. [DOI] [PubMed] [Google Scholar]
- Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis. 2006;6:55. doi: 10.1186/1471-2334-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


