FIGURE 1.
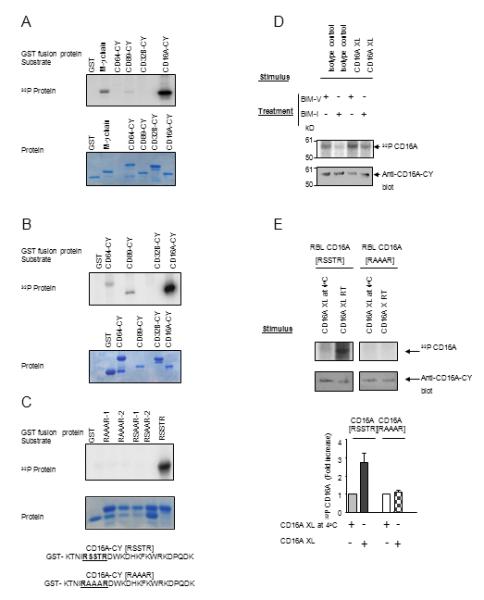
CD16A is specifically phosphorylated by PKC. (A) and (B) In vitro kinase assay using PKCı (A) and PKCαβγ (B) with various GST-Fc receptor CY domains (upper panel). Protein gel was stained with Coomassie Blue (lower panel). Data are representative of six experiments. (C) Cytoplasmic domain sequences of wild type ([RSSTR]) and the mutant ([RAAAR]) CD16A. IVK substrates include separate clones of CD16A-CY with S/T to A mutations within the [RSSTR] motif (RAAAR-1 and -2, RSAAR-1 and -2 represent duplicate assays). Protein gel was stained with Coomassie Blue (lower panel). Data are representative of three separate experiments using PKCδ. (D) CD16A-stably transfected P388D1 cells were metabolically labeled with [32P] orthophosphate and treated with 10ug/mL BIM-I or control reagent BIM-V at 37°C for 60 minutes prior to receptor cross-linking for 5 min at 25°C. Immunoprecipitated CD16A was detected by autoradiogram and parallel gels blotted with rabbit anti-CD16A-CY. Data are representative of three experiments. (E) [RSSTR] or [RAAAR] CD16A-stably transfected RBL-2H3 cells were metabolically labeled with [32P] orthophosphate and receptor was cross-linked for 5 min at 25°C. Immunoprecipitated CD16A was analyzed by autoradiography, western blotting, and densitometry (upper panel). The density of [32P] CD16A was plotted for each treatment condition (lower panel). Data are means of three experiments.
