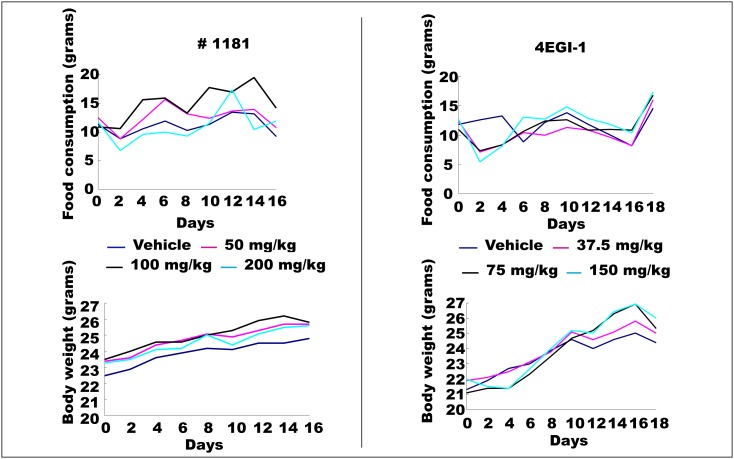
Figure 5. Maximum tolerated dose (MTD) assay for #1181 and 4EGI-1.
MTD was assessed by injecting groups of 5 male and 5 female nude mice with different intra-peritoneal (i.p.) doses of each compound for 5 consecutive days followed by observation for additional 15 days, in accord with NIH protocols. At the concentrations used, injection of either compound did not result in significant weight loss, reduced daily food intake, behavioral changes or any other observable sign of toxicity.