Table 3. Investigation of substrate scope for the SGP-catalysed asymmetric Mannich reactiona.
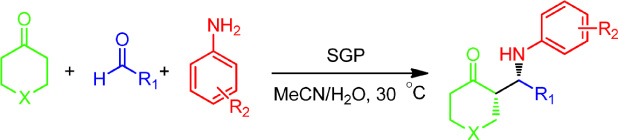 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | X | R1 | R2 | Product No. | Time (h) | Yield (%)b | dr (syn:anti)c | e.e. (syn) (%)d |
| 1 | CH2 | 4-NO2C6H4 | H | 4a | 96 | 64 | 88:12 | 83 |
| 2 | CH2 | 4-CF3C6H4 | H | 4b | 94 | 73 | 81:19 | 78 |
| 3 | CH2 | 4-BrC6H4 | H | 4c | 94 | 65 | 74:26 | 76 |
| 4 | CH2 | 4-ClC6H4 | H | 4d | 120 | 92 | 78:22 | 75 |
| 5 | CH2 | 3-FC6H4 | H | 4e | 94 | 72 | 70:30 | 74 |
| 6 | CH2 | 4-FC6H4 | H | 4f | 94 | 66 | 66:34 | 64 |
| 7 | CH2 | 4-CNC6H4 | H | 4g | 120 | 61 | 58:42 | 68 |
| 8 | CH2 | C6H5 | H | 4h | 100 | 62 | 60:40 | 61 |
| 9 | CH2 | 4-CH3C6H4 | H | 4i | 120 | 68 | 40:60 | 33 |
| 10 | CH2 | 4-NO2C6H4 | 3-Br | 4j | 144 | 24 | 92:8 | 88 |
| 11 | CH2 | 4-NO2C6H4 | 3-CH3 | 4k | 123 | 54 | 91:9 | 83 |
| 12 | CH2 | 4-NO2C6H4 | 4-Cl | 4l | 144 | 47 | 89:11 | 83 |
| 13 | CH2 | 4-NO2C6H4 | 4-CH3 | 4m | 120 | 81 | 90:10 | 82 |
| 14 | CH2 | 4-NO2C6H4 | 4-OCH3 | 4n | 117 | 71 | 72:28 | 72 |
| 15 | CH2 | 4-BrC6H4 | 4-OCH3 | 4o | 165 | 66 | 52:48 | 40 |
| 16e | S | 4-ClC6H4 | H | 4p | 168 | 81 | 57:43 | 58 |
| 17e | S | 4-CF3C6H4 | H | 4q | 142 | 80 | 44:56 | 52 |
aReaction conditions: a mixture of aromatic aldehyde (0.5 mmol), arylamine (0.55 mmol), ketone (7.5 mmol), deionised water (0.10 mL), MeCN (0.9 mL) and SGP (50 mg) was stirred at 30°C.
bYield of the isolated product after silica gel chromatography.
cDetermined by chiral HPLC analysis.
de.e. value of the syn-isomer, determined by chiral HPLC; the absolute configuration was assigned by comparison with the literature (for details, please see the Supplementary Information).
etetrahydrothiopyran-4-one (1 mmol).
