Abstract
The conserved Notch signaling pathway plays crucial roles in developing and self-renewing tissues. Notch is activated upon ligand-induced conformation change of the Notch negative regulatory region (NRR) unmasking a key proteolytic site (S2) and facilitating downstream events. Thus far, the molecular mechanism of this signal activation is not defined. However, strong indirect evidence favors a model whereby transendocytosis of the Notch extracellular domain, in tight association with ligand into the ligand-bearing cell, exerts a force on the NRR to drive the required structure change. Here, we demonstrate that force applied to the human Notch2 NRR can indeed expose the S2 site and, crucially, allow cleavage by the metalloprotease TACE (TNF-alpha-converting enzyme). Molecular insight into this process is achieved using atomic force microscopy and molecular dynamics simulations on the human Notch2 NRR. The data show near-sequential unfolding of its constituent LNR (Lin12-Notch repeat) and HD (heterodimerization) domains, at forces similar to those observed for other protein domains with a load-bearing role. Exposure of the S2 site is the first force “barrier” on the unfolding pathway, occurring prior to unfolding of any domain, and achieved via removal of the LNRA∶B linker region from the HD domain. Metal ions increase the resistance of the Notch2 NRR to forced unfolding, their removal clearly facilitating unfolding at lower forces. The results provide direct demonstration of force-mediated exposure and cleavage of the Notch S2 site and thus firmly establish the feasibility of a mechanotransduction mechanism for ligand-induced Notch activation.
Keywords: mechanical force, signaling receptor
The Notch signaling pathway (1, 2), though simple and direct compared with many signaling pathways, has a diverse and crucial role in both developing tissues and adult tissues that undergo self-renewal. The pathway functions by enabling cell–cell communication that, depending on signal context and dose, directs the cells to a preferential cell fate. Through this system, the pathway regulates numerous cellular decisions including cell cycle progression, metabolism, and cell differentiation (3–5). With such a diverse and crucial role in development and self-renewal, it is unsurprising that mutations and errors within this signaling pathway give rise to several diseases and cancers (6–10).
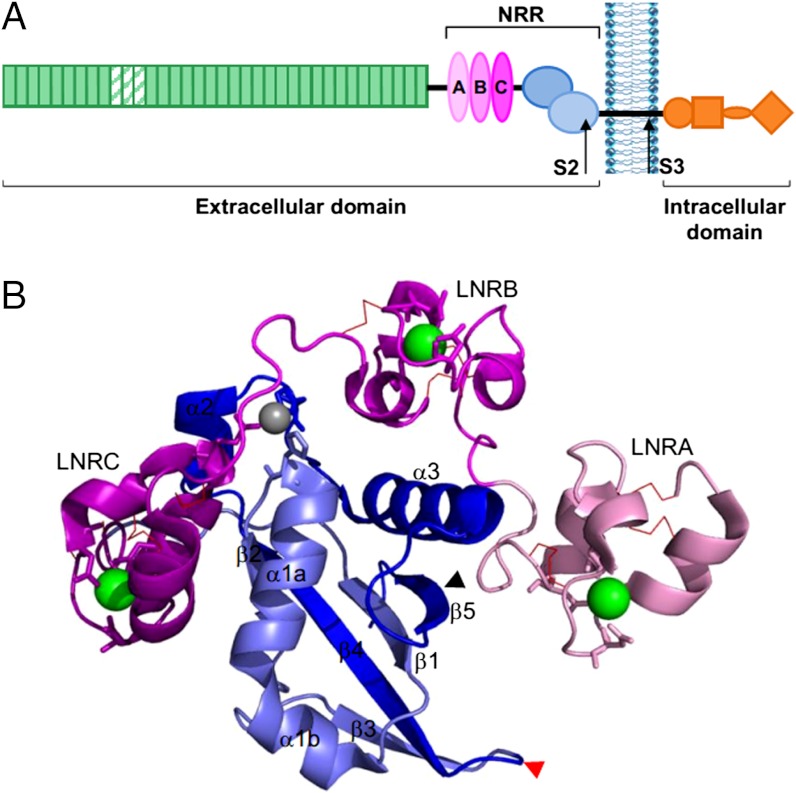
In humans, there are four Notch receptors, Notch1–4, which are very similar in domain architecture and all require proteolytic processing for signal activation and transmission via the canonical pathway. Prior to signal activation, a Notch receptor is expressed on the cell surface as a furin-cleaved heterodimer (furin cleaves at site S1). A second proteolytic cleavage site, S2, is buried within the fold of the extracellular Notch negative regulatory region (NRR; Fig. 1) (11, 12), where it is protected from cleavage by certain proteases of the ADAM (a disintegrin and metalloprotease) family (13, 14). The binding of transmembrane ligand (Delta/Serrate/Lag-2) (15, 16), presented on a neighboring cell, to certain Notch epidermal growth factor (EGF)-like repeats, in some way triggers cleavage at S2. Once cleaved at this site, γ-secretase can access a third site (S3) in the Notch transmembrane region, cleaving and consequently releasing the Notch intracellular domain, which translocates to the nucleus to upregulate its target genes. Insight into the mechanism by which ligand binding results in a structure change in the Notch NRR, sufficient to facilitate S2 cleavage, is key to understanding the activation process.
Fig. 1.
The Notch receptor and the NRR of hN2. (A) The domain structure of the Notch receptor contains an extracellular region, consisting of the NRR and the EGF-like repeats (green; repeats required for ligand interactions are striped). The NRR comprises three LNR modules (light pink to dark pink) and the furin-cleaved HD domain (HD-N, dark blue; HD-C, light blue). Approximate positions of S2 and S3 cleavage sites, which release the intracellular domain, are shown. (B) X-ray crystal structure of hN2-NRR in its autoinhibited conformation. The LNR and HD domains are colored as in A, each LNR containing a coordinated calcium ion (green) and three disulfide bonds (red lines). The zinc ion, coordinated by residues of the B∶C linker and HD domain, is shown in gray. The S2 cleavage site is highlighted by a black arrow. The furin cleavage loop (S1) is indicated by a red arrow. PDB ID code 2OO4 (12). Figure created using PyMol (version 1.3).
Crystal structures of the NRR region of the human Notch1 (17) and Notch2 (12) receptors show burial of the S2 site via wrapping of the three LNR (Lin12-Notch repeat) modules (A–C) around the HD (heterodimerization) domain, with the connecting linker between LNRA and B (A∶B) forming a plug of inhibition over the S2 cleavage site in the HD domain. Each LNR domain is stabilized by three disulfide bonds and a coordinated calcium ion. In the human Notch2 structure, a zinc ion is also present close to the linker of LNRB and C (B∶C) (Fig. 1). Because the key region for ligand binding (EGF-like repeats 11–13) (18, 19) is distal to the S2 cleavage site by a further approximately 20 EGF-like repeats in human Notch1–4, and significant parts of the EGF repeat region will be in an extended rod-like structure (20), an allosteric mechanism for conformational change at S2 would seem unlikely and, indeed, has little experimental support. Conversely, considerable evidence now exists that Notch relies on endocytosis of its ligand for signal activation in the receiving cell. A number of studies in vertebrates and flies have proposed that this ligand endocytosis provides a mechanical force to unravel at least part of the Notch NRR structure. Firstly, the forces of adhesion and dissociation measured between Notch-bearing and ligand-bearing cells indicate an extremely tight Notch-ligand association that is proportional to signaling rates (21). Indeed, somewhat crucially, transendocytosis of the Notch extracellular region with ligand into the ligand-presenting cell has been observed (22, 23). Further indirect evidence for force-mediated Notch activation comes from observations that Notch signaling is enhanced upon immobilization of soluble forms of ligand and that, if not immobilized, such soluble ligands actually inhibit Notch signaling (24–26).
The role of mechanical force in cellular signaling mechanisms is an emerging and rapidly expanding area (27). The initial sensing of a mechanical stimulus (28–30) occurs at a cell surface, and among identified “sensors” are cell adhesion molecules, integrin receptors, ion-sensitive channels, protein kinases, and G proteins (31–34). Although there is increasing evidence for mechanically regulated signaling pathways, including the Notch pathway studied here, direct evidence that provides insight into the mechanosensors and signal transduction events at a detailed molecular level is lacking. This lack of molecular detail is in part due to the challenges of applying force techniques on biological systems, which will require staged approaches and combined technologies. This work represents experimental application of force on a Notch molecule, with observation of effect, aimed at directly probing the feasibility of a force-induced protein unfolding mechanism for exposure of the S2 site within the NRR. We use recombinant NRR from human Notch2 (hN2), for which the crystal structure first revealed an autoinhibited conformation (12). Using atomic force microscopy (AFM), we monitor unfolding of the constituent LNR and HD domains of the hN2-NRR, validating these experimental observations via steered molecular dynamics (MD) simulations, which provide further molecular insight into structural changes as the NRR unfolds. The combination of techniques yields the greatest molecular insight thus far into the response of the Notch2 NRR to an applied force, such as it presumably experiences upon transendocytosis. Importantly, we correlate NRR mechanical unfolding to S2 site accessibility by the metalloprotease TACE (TNF-alpha-converting enzyme). Direct observation of cleavage at S2 upon mechanical stretching of Notch is observed.
Results
Characterization of the Mechanical Properties of hN2-NRR by AFM.
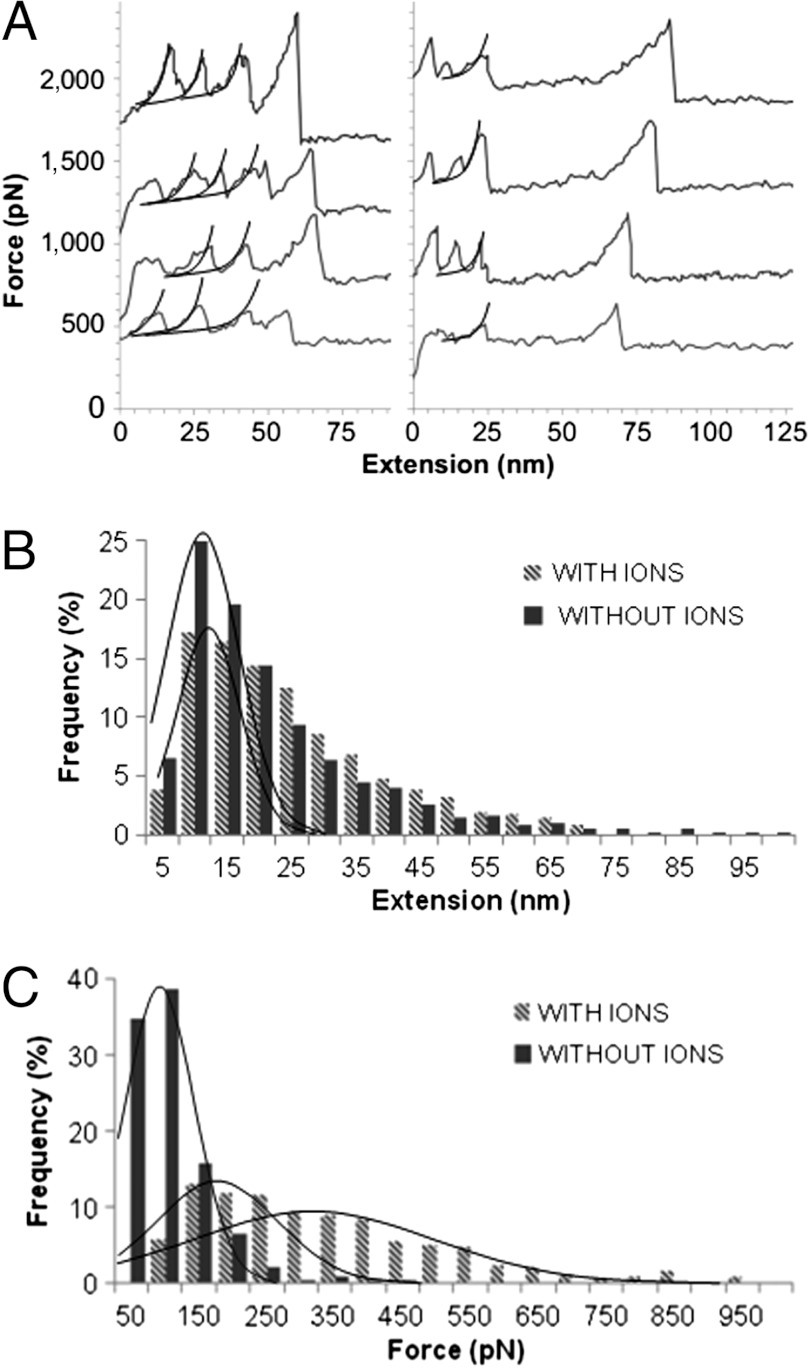
Mechanical unfolding of the Notch NRR has been hypothesized to expose the S2 cleavage site, allowing metalloprotease access and cleavage that is required for signal pathway activation. To determine the mechanical stability of the NRR of the human Notch2 receptor, AFM was performed on recombinant hN2-NRR (hN2 residues 1425–1672), comprising the LNR modules and HD domain. AFM is used to apply force by pulling on single protein molecules at a constant loading rate, with the unfolding of a protein domain corresponding to a peak in the observed force as the molecule is extended. The hN2-NRR protein construct contained an amino-terminal poly-lysine tag to optimize directed covalent attachment of this protein terminus to an N-hydroxysuccinimide (NHS)-functionalized gold surface. A carboxy-terminal hexahistidine tag was used to direct attachment of the AFM tip (functionalized with Ni2+-NTA) to the C terminus of the protein. Although a protein construct comprising NRR polymeric repeats would simplify data interpretation (35, 36), a single NRR contains 10 disulphide bonds, presenting a formidable challenge (which we could not overcome) to obtaining correctly folded polymeric product by either tandem cloning or chemical ligation routes. Hence, a single recombinant hN2-NRR stabilized by disulfide bonds and coordinating metal ions in the correctly folded conformation, as assessed by CD and two-dimensional 1H NOESY NMR, was attached between AFM cantilever tip and slide and subjected to a pulling force in the AFM. Notably, the hN2-NRR protein does in itself contain multiple domains. Representative saw-tooth force-extension curves observed upon stretching hN2-NRR single molecules by AFM (Fig. 2A) show features consistent with unfolding of the multiple domains within this protein fragment. In force-extension curves, at least two smaller extensions, to approximately 7 nm, were repeatedly observed. Extension of LNRs to the full theoretical maximum (for 35–40 residues) of approximately 10.5–12 nm is rarely observed due to the three disulphide bonds present in each LNR. Peaks in the saw-tooth profiles with larger force or extension features are attributed to the HD domain, for which full extension to the theoretical maximum (55 nm) is also not observed. A slight shortening of this domain is expected due to the presence of a stabilizing disulfide bond. However, the range of extensions observed also indicates some nonspecific attachment of the NHS linker to lysine side chains in the HD domain. Histogram analysis of the observed extensions demonstrates a unimodal non-Gaussian distribution of the unfolding lengths of the NRR (Fig. 2B), where the peak correlates to LNR unfolding between 5 and 15 nm (mode 6.88 nm). The trailing tail of the distribution is due to the aforementioned attachment to lysine residues outside of the polyK-tag, resulting in the lack of a distinctive peak for the HD domain. Forces required for NRR unfolding show a bimodal distribution (median = 179 pN and 372 pN; Fig. 2C), consistent with the unfolding of two types of domain structure. Notably, the forces measured fall within ranges observed for proteins with a load-bearing mechanical role (37–39). The loading rate used for force measurements was 1 × 10-7 N/s (spring constant always within 10% of 67 pN nm-1). As expected for unfolding of protein domains, force values associated with the recorded curves changed linearly with the logarithm of the pulling speed (40) when this speed was altered.
Fig. 2.
AFM unfolding of hN2-NRR region. (A) Raw data from AFM experiments with NRR showing distinct unfolding features corresponding to the LNR (Left) and HD domains (Right). Worm-like chain (WLC) analysis shown on unfolding curves (black line). Mean coefficient of determination for WLC analysis of data = 0.98. The first peak was shown (via controls) to correspond to salt contacts between the tip and slide, and is therefore not analyzed by the WLC. Frequency of extension (B) and force (C) observed during all unfolding events (LNR and HD) in the presence (striped; n = 1,075) and absence (block color; n = 866) of coordinated ions (x axis values represent upper limit of histogram interval). Curve on extension histogram highlights the peaks for LNR domains unfolding. Distinct HD domain unfolding not observed (expected extension 55 nm). Variations due to nonspecific attachments to the hN2-NRR construct outside the polyK tag produce background features over a large range of extensions. Curves on the force histogram highlight bimodalality and therefore unfolding of two species. In the absence of ions, this bimodal feature is lost and the force required for unfolding is reduced.
Previous research has shown the removal of coordinated metal ions leads to an open NRR structure with a more easily accessible S2 cleavage site (41) as well as allowing ligand-independent signaling activation (41, 42). Furthermore, there is evidence that the level of Notch signaling is sensitive to levels of extracellular calcium in vivo (43). It is thus pertinent to examine the influence of coordinated metal ions on the mechanical unfolding of hN2-NRR. The differences observed in AFM experiments on protein constructs containing and lacking (EDTA-treated) coordinated ions are shown in Fig. 2B and C. Although a little difference is observed in the unfolding lengths of these domains, the forces required for unfolding are dramatically lowered when coordinated ions are removed. Also, the bimodality of the force distribution is lost when ions are removed, shifting the peaks of median 179 and 372 pN (ions present) to 65 pN (ions removed). The large reduction in force required for unfolding and loss of the characteristic distribution suggest the coordinated ions make a significant contribution to the structural integrity of the NRR.
We also examined the influence of the disulfide bonds on the force-extension profiles by repeating the AFM experiments in the presence of DTT. Histogram analysis of the data (Fig. S1) shows a marked increase in the extensions (median: wild type = 18.9 nm, DTT treated = 38.4 nm), while the force only slightly decreases (median: wild type = 309.2 pN, DTT treated = 238.2 pN). The increase in observed extension lengths correlates with lack of observed extension of the LNRs, allowing us to more confidently assign the histogram peaks in standard experiments to LNR and HD domains, respectively.
MD Simulations Show S2 Cleavage Site Exposure in hN2-NRR Corresponds to Removal of the Linker Region Between LNRA and LNRB.
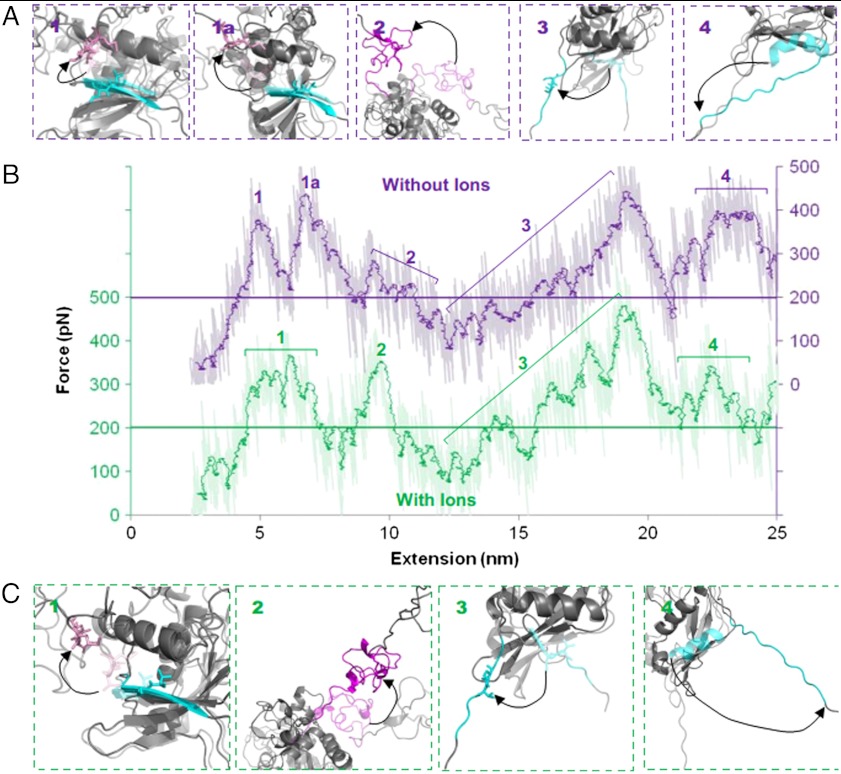
To gain a greater understanding of the unfolding process that occurs when force is applied to the NRR, and to strengthen interpretation of the AFM data, MD simulations were performed using GROMACS (see Materials and Methods). Simulations were carried out using the crystal structure of the hN2-NRR both in the presence and absence of coordinated ions [Protein Data Bank (PDB) ID code 2OO4] (12), with application of a constant loading rate (1 N/s) on the atoms of the N-terminal residue (Cys1425) while the C-terminal residue (Thr1672) is positionally restrained. The video output from the forced unfolding simulation is shown in Movie S1. Fig. 3 shows the changes in solvent accessible surface area (SAS) at the S2 cleavage site as the domains unfold, as well as analysis of distances between defined secondary structure elements. Fig. 4 shows differences in the force-extension profiles produced in the presence and absence of ions (curves shown are representative of multiple obtained through repeated MD simulations). Both Figs. 3 and 4 show snapshots of the unfolding protein structures associated with labeled transitions. Changes in the SAS of the S2 cleavage site provide a quantitative measure of the accessibility of this region to metalloprotease cleavage. The largest increase in the SAS of the S2 site occurs between 300 and 1,000 ps. When combined with simulation snapshots and the distance analyses (Fig. 3), it is evident that this increase results from the removal of the A∶B linker from the S2 cleavage site. Correlation analysis of the SAS over this time and the distance between the A∶B linker and the S2 cleavage site shows strong correlation in all simulations performed (correlation coefficients between 0.76 and 0.80). Furthermore, the removal of the A∶B linker from the cleavage site event corresponds to the first major feature (peak 1) seen on a typical force extension curve (Fig. 4), which reaches a force of approximately 375 pN at an extension of around 6.5 nm. Further unfolding, specifically the removal of the S2 cleavage site from the surrounding HD domain, causes smaller increases to the SAS at the S2 cleavage site while requiring similar levels of force (peak 3, Fig. 4). The remaining peaks within Fig. 4 correspond to the unfolding of the LNRB from the HD domain (peak 2) and the unraveling of the α3 helix (peak 4).
Fig. 3.
Comparison of the changes in SAS of S2 cleavage site with the changes in distance between domains from MD simulations. (A) Differences in the distances between domains in the presence (dark) and absence (light) of coordinated ions do not affect the SAS of the S2 cleavage site (gray). (B) Screen shots of the corresponding changes in the NRR domain structure as unfolding occurs. Key changes are observed in the β5 strand housing the S2 cleavage site (blue; relevant side chains shown), and the LNR A∶B linker (pink; relevant side chains shown). Starting screenshot for each SAS increase shown semitransparent; arrows highlight change in structure. Data generated from GROMACS 4.5.3; images created in PyMol (version 1.3).
Fig. 4.
Force-extension output from MD simulations comparing differences in the unfolding profile of the LNR-HD region in the presence and absence of ions. Force-extension graphs (B) comparing the absence (purple) and presence (green) of ions are staggered by 300 pN. The horizontal line dissecting the plots highlights the 200 pN point on both curves, raw data shown as well as a running average (period: 50). Screenshots for each highlighted peak are shown for simulations in the absence (A) and presence (C) of coordinated ions. Structure before unfolding peak is shown semitransparent and main features highlighted with color; arrows show key changes. Data generated from GROMACS 4.5.3; images created in PyMol (version 1.3).
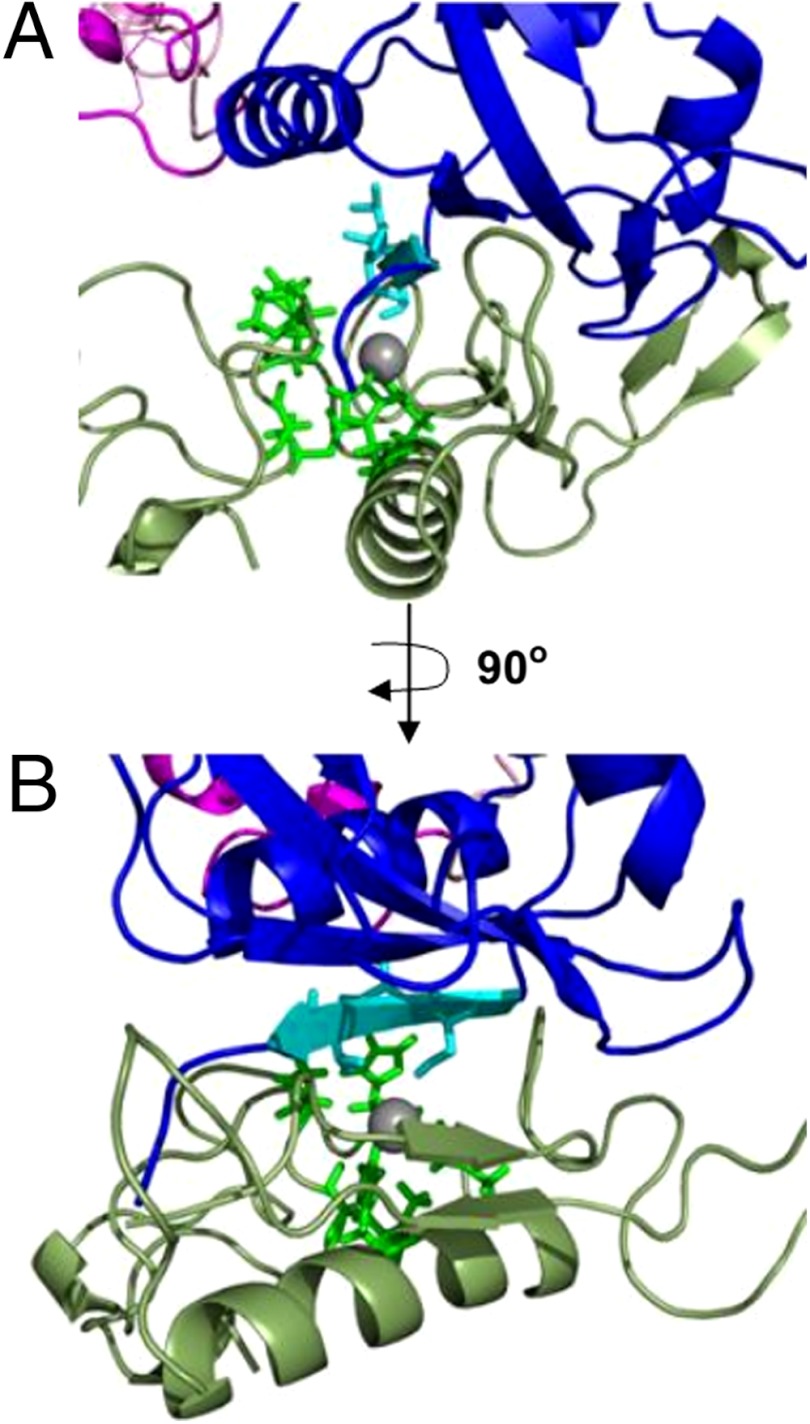
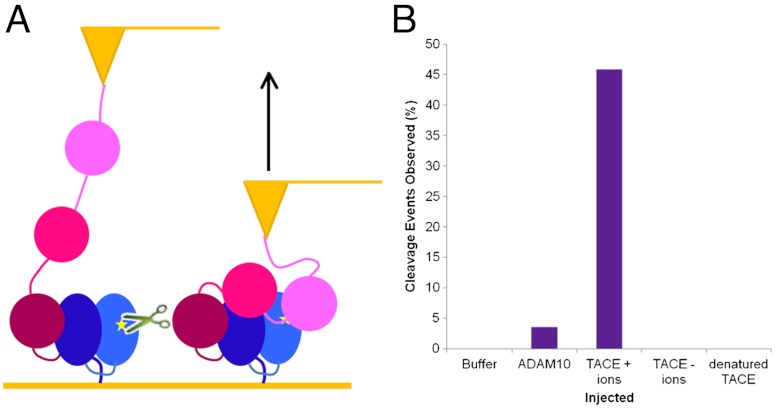
To determine whether exposure of the S2 site, through the removal of the A∶B linker, could allow access to metalloproteases, the partially unfolded NRR was manually docked into the active site of TACE (44) (Fig. 5). The S2 site of the partially unfolded construct fits nicely into the active site cleft of TACE with no steric hindrance from the surrounding structure. Hence, MD simulations combined with molecular docking demonstrate that, after only small amounts of unfolding, the NRR structure is in a conformation providing access for metalloproteases known to cleave it.
Fig. 5.
Manual docking simulation of partially unfolded NRR in TACE active site. NRR from unfolding simulation (time point: 1,000 ps; following unfolding event 1 in Fig. 4C). (A) β5 strand housing the S2 cleavage site (light blue) fits snugly into the binding pocket of the TACE active site (moss green). Zinc binding residues and conserved catalytic residues are shown in lime green; coordinated zinc ion is shown in gray. (B) Rotation of 90° shows no steric hindrance from the HD domain (dark blue) at this early stage of unfolding. Docking performed with PyMol (version 1.3); TACE PDB ID code 1BKC (44).
Metal Ions Influence the Stability and Unfolding of the hN2-NRR.
In AFM experiments, a notable reduction in forces for mechanical unfolding of hN2-NRR was observed in the absence of metal ions, indicative of a major role for these ions (Ca2+ and Zn2+) in guiding the structural integrity of this region of Notch. The influence of metal ions was therefore examined further via simulation. When coordinated ions were removed from the MD simulations, four key changes were observed. Firstly, unfolding of the domains takes slightly longer when ions are removed. This first becomes apparent when the A∶B linker is pulled away from the S2 cleavage site (Fig. 3). When the ions are removed, a delay is seen for this unfolding event. Examination of the simulation output shows LNRA extending further when the calcium ions are missing, causing a delay in the time it takes for the A∶B linker to be placed under force. Furthermore, when ions are present, the distance between the A∶B linker and S2 cleavage site stalls at approximately 3.5 nm for 300 ps. This stall of unfolding occurs before the LNRB structure is removed from the HD domain and is caused by the lower extension (retention of more structure) of LNRA when ions are coordinated, leading to a more prominent stall before a force magnitude is reached for LNRB unfolding to occur. An increase in time required for separation of LNRB from the HD domain and for α3 helix unfolding and pulling away from the β1 strand of the HD domain is also observed in the absence of metal ions. These differences can also be linked to the shortened time scale of the LNR domain unfolding when ions are present.
Secondly, the forces required for removing the A∶B linker from the S2 cleavage site are affected by metal ions (Fig. 4). When coordinated ions are present, the force required for the removal of the A∶B linker from the S2 site forms one broad peak at 375 pN spanning approximately 2.5 nm (peak 1, green) whereas, when these ions are removed, the barrier clearly separates into two force peaks (peaks 1 and 1A, purple). These peaks span approximately the same distance, although with a slight delay when ions are removed. The first of these two peaks corresponds to the unplugging of the S2 cleavage site and reaches a force of approximately 375 pN, comparable to the forces seen when ions are present. The second peaks at a force of around 450 pN and appears to be due to the methionine residue within the A∶B linker forming intermediate contacts with the side chains of the residues within the α3 helix.
The third effect of metal ion removal, which in this case shows a change in force magnitude, occurs to the peak at approximately 10 nm extension (Fig. 4B; peak 2, with and without ions). This peak, which represents a barrier of approximately 200 pN in the presence of ions, is reduced to a smaller undefined peak when ions are absent. From examination of the screenshots, the force event corresponds to the removal of LNRB from the HD domain. This event is thus particularly sensitive to the presence of metal ions during the simulated mechanical unfolding pathway. Interestingly, a zinc ion is located between the B∶C linker and the HD domain. Although an effect of calcium ions on increasing the mechanical stability of individual LNR modules can be rationalized and offered in explanation of differences in their unfolding timescales (Fig. 3), the zinc ion is better positioned for a specific effect on disruption of the LNRB∶C linker–HD interaction.
Finally, where coordinated ions are present, unfolding for the LNRC is not observed during the timescale of simulation. When coordinated ions are absent, the LNRC begins to pull away from the HD domain following β5 strand removal and α3 unfolding. The partial unfolding of this domain when the coordinated zinc and calcium ions are absent again suggests a stabilizing role for these ions.
Overall, removal of metal ions reduces the structural stability of the NRR, with a newly demonstrated role for zinc in stabilizing the LNR B∶HD and LNR C∶HD interface. The simulated mechanical unfolding data concurs with the reduced forces for mechanical unfolding of hN2-NRR observed by AFM in the absence (versus presence) of metal ions.
Mechanical Unfolding of hN2-NRR Allows Metalloprotease Cleavage at the S2 Site.
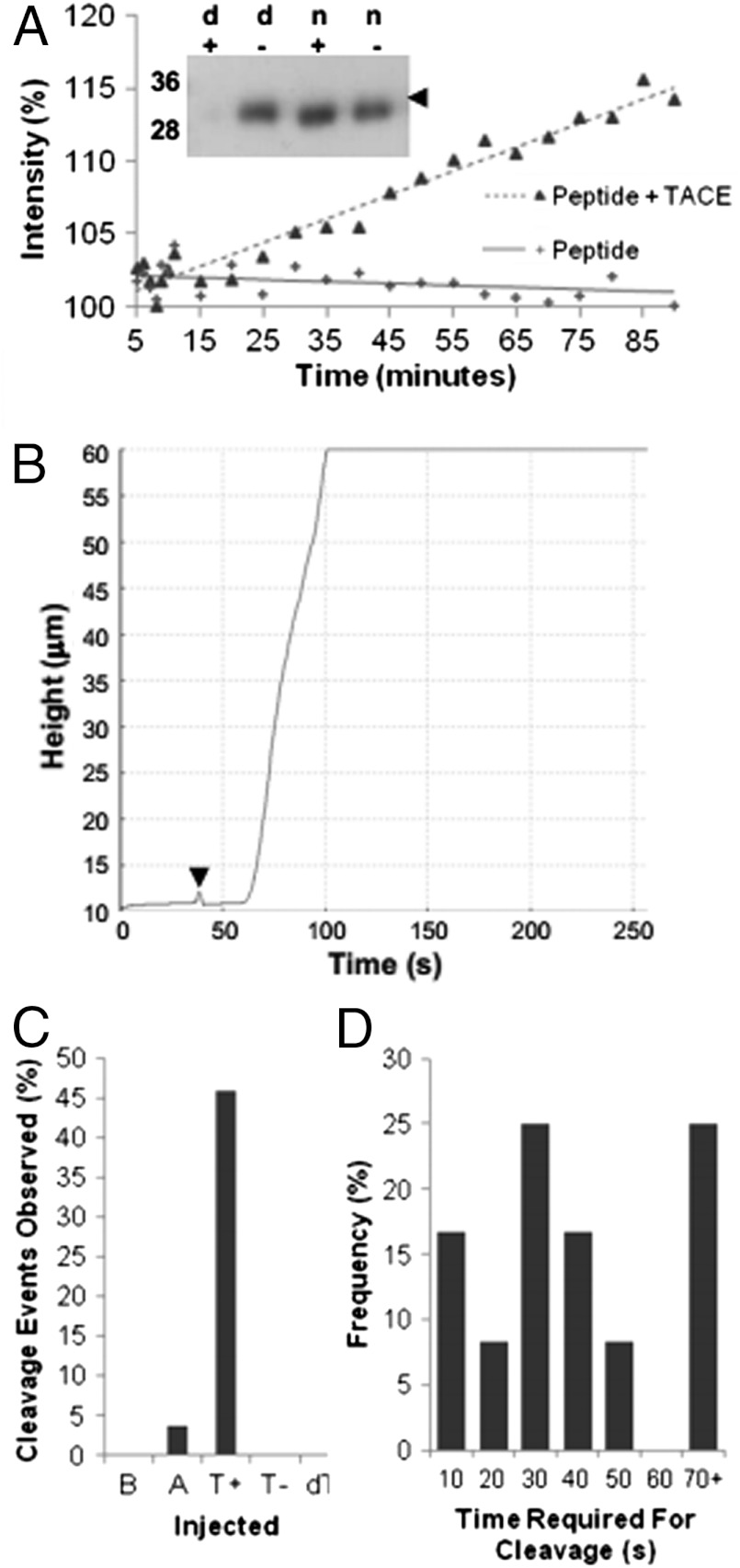
Because simulation demonstrates that the S2 site is exposed early in the unfolding pathway of the NRR, experiments were performed to determine whether mechanical unfolding could lead to observation of cleavage at the S2 site. Initial experiments were performed to ensure that the TACE enzyme shows activity for unfolded NRR in vitro. Firstly, cleavage assays were performed using a quenched fluorescent peptide substrate, homologous to the S2 cleavage site of hN2. On incubation with TACE, an increase in fluorescence was seen compared with the peptide alone, showing cleavage of the peptide had been achieved (Fig. 6A). Note that cleavage is specific, cleaving prior to the two Val residues as expected as determined by mass spectrum analysis and N-terminal sequencing (Fig. S2). Analysis of the enzyme specificity was then performed on the recombinant hN2-NRR structure. Because the S2 site is housed near the C terminus of the protein, cleavage will remove the His tag from the protein, within an 18 amino acid (2.2 kDa) fragment. Given the small percentage change in the molecular weight of N2-NRR that removal of this fragment causes, changes in stained protein band migration analyzed by SDS-PAGE are not conclusive. A Western blot was thus performed with antibodies raised against the C-terminal His6 tag (Fig. 6A, Inset) for protein samples that were folded or denatured and treated or not treated with TACE. The denatured construct treated with TACE shows a loss of signal, compared with untreated denatured construct, and TACE treated “native” construct. In essence, TACE cleavage is specific to the hN2 S2 cleavage site and cleaves only when the protein is in the denatured form. The results confirm S2 site burial within the folded native protein and its exposure for TACE cleavage in the denatured protein. The same experiments were performed using ADAM10, another metalloprotease known to cleave Notch in vivo, and also demonstrated successful cleavage, albeit to a somewhat lower degree (see Fig. S3).
Fig. 6.
Cleavage experiments using TACE to determine unmasked S2 site specificity and whether mechanical force can allow for S2 cleavage. (A) Cleavage is observed when TACE is incubated with hN2 S2 site fluorescent peptide. Increase in fluorescence over time after addition of TACE shows cleavage occurring at S2 site. Western blot (Inset) probed for the C-terminal His-tag of recombinant protein shows a loss of signal in the denatured protein (d) containing TACE (+) highlighting it’s specificity for the unfolded form of the protein. Controls lacking TACE (-) or containing nondenatured protein (n) show no cleavage. (B) Raw data from AFM cleavage experiments. The height of the AFM tip is measured over time; when protein is held, the tip is maintained close to the surface; however, when cleavage occurs tension across the tip is lost and retraction from the surface is observed. Point of TACE injection is marked by arrowhead. (C) Graph showing percentage frequency of cleavage events when zinc chloride containing buffer (B, n = 65), ADAM10 (A, n = 56), TACE in a zinc chloride buffer (T+, n = 61), TACE without the necessary zinc ions (T-, n = 54), and denatured TACE (dT, n = 72) are injected. (D) Graph showing time taken postinjection for cleavage events to occur when TACE was injected (x axis values represent the upper limit of the histogram interval).
Having established activity of TACE on hN2-NRR in vitro, AFM experiments were set up to maintain a constant force of 200 pN across the construct for 5 min. This level of force is sufficient to unfold LNRA and LNRB, and the S2 site should therefore have become exposed. Lower forces were not possible in this mode because of the difficulty in distinguishing attachments from salt contacts. During this 5-min clamp at constant force, varying substances were injected in close proximity to the construct. Essentially, if cleavage occurred during this clamp there would be a loss of tension between the tip and the surface of the sample, resulting in retraction of the tip (Fig. 6B). In experiments where buffer alone was injected, no cleavage events were observed (Fig. 6C). When TACE was injected without the necessary metal ions, or when denatured TACE was injected, no cleavage events were observed. In comparison, when TACE and the necessary metal ions were injected, 46% of force clamps showed cleavage occurring. The majority of the TACE cleavage events occur between 20 and 40 s after the point of injection; however, some have taken up to 190 s (Fig. 6D). The methodology relies upon diffusion of the enzyme toward the clamped substrate within a relatively short time frame at a lower than optimum temperature. Given the nature of the experiment, the percentage of the recorded extension curves that show loss of tension and tip retraction due to N2-NRR cleavage when TACE is injected is significant. Injection experiments were also performed with ADAM10. Although this enzyme preparation is less active in vitro and is certainly more reliant on incubation at 37 °C, it does show some ability to cleave during the force clamp (which could only be performed at room temperature), with 3.6% of force clamps showing cleavage events.
Discussion
It is now accepted that activation of the Notch signaling pathway relies on the S2 cleavage event (45). However, the mechanism by which this cleavage is induced upon ligand binding has remained a subject for speculation. In its resting state, the hN2-NRR is in an autoinhibited conformation (12), with a clear requirement for a change in structure to unmask the S2 site. A mechanotransduction hypothesis is favored, in which the required conformational change results from a mechanical force produced from the transendocytosis of the Notch extracellular domain into the ligand presenting cell. Despite indirect evidence for this hypothesis, no direct experimental evidence yet exists to demonstrate that a mechanical force applied to a Notch molecule could actually unmask the S2 site. Here, we have shown, through the use of AFM and MD simulations, that the S2 site of hN2-NRR is revealed with minimal mechanical unfolding, allowing for cleavage at this site. Our research thus provides direct evidence on a molecular level to support the feasibility of a mechanotransduction mechanism for Notch signal activation.
AFM experiments show that the forces required for NRR unfolding follow a bimodal distribution with peaks corresponding to unfolding forces with medians of 179 and 372 pN. The lower force peak is associated with unfolding of individual small LNR modules and the latter probably with the larger globular HD domain (or a combination of this domain and LNRC, see below). The recorded forces fall within the range required for unfolding of immunoglobulin domains of mechanically stable titin-like proteins (37–39), rather than nonmechanosensing proteins such as GFP and barnase (46, 47). The domains within the hN2-NRR region will thus clearly offer resistance to mechanical stimuli, in keeping with a mechanosensing role.
All-atom steered MD simulations were performed to provide greater insight into the mechanism behind the unfolding process occurring when the Notch NRR is subject to force. Although these simulations run at speeds of several orders of magnitudes faster than in the AFM experiments, due to computational limitations, and thereby give higher force measurements, such methods can correctly predict mechanical unfolding pathways and certainly aid interpretation of experimental data (48, 49). The four domains of the NRR exhibit a largely but not entirely sequential pattern of unfolding during MD simulation. Although the first two LNR modules unfold first, the β5 strand emerges from the HD structure, followed by further HD domain partial unraveling, without the prior requirement for LNRC unfolding. Because LNRC unfolding is not observed in the period of our simulation, the forces required for the unfolding of this domain are much larger compared with the forces needed to unfold LNRA and LNRB. Therefore, we can more confidently conclude that lower forces seen during LNR unfolding in AFM experiments correspond to the unfolding of the first two LNRs. Furthermore, larger force peaks in the AFM data, attributed to the HD domain, might also be a combination of HD domain and LNRC unfolding. Importantly, MD simulations reveal that the S2 cleavage site is exposed early in the mechanical unfolding pathway, after the first main structure change that “unplugs” LNR A∶B linker from its position over the S2 site (located in the β5 strand of the HD domain). Removal of the “plug” formed by the A∶B linker produces a large increase in the solvent accessibility of the S2 cleavage site. Extraction of the β5 strand, housing the S2 site, from the HD domain requires similar forces to those seen for the removal of the A∶B linker but produces only a minimal increase to the SAS, calling into question its importance in relieving autoinhibition. Indeed, manual docking, performed after the removal of the A∶B linker, shows that the β5 strand bearing the S2 site can be accommodated in the TACE active site cleft, without disruption to the HD domain structure. Note that these findings are different to those reported upon coarse grain simulation of the unfolding of the hN1-NRR (50), which show a perfect sequential removal of all three LNR domains from the HD structure and further propose that some unraveling of the HD domain is potentially required for metalloprotease cleavage at S2. Although the unfolding pathways revealed by MD simulation differ slightly for N1 versus N2-NRR, it is difficult to be certain whether this difference is a reflection of the different simulation methods used or the different protein subject. Notably, though, the hN1-NRR does not contain a zinc ion located between the HD domain and the LNRB-C linker region. Both AFM and the MD simulations on hN2-NRR demonstrate metal ion-dependent structural stability of the NRR region. Moreover, our simulations highlight a role for the zinc ion in increasing the stability of the HD domain∶LNRC interaction through its interactions with residues from the B∶C linker, LNRC, and the HD domain, thereby influencing the unfolding pathway. To date, there is a lack of experimental data for N1-NRR unfolding, though a preliminary AFM study on the mouse N1-NRR has recently been published that reports similar sizes of unfolded LNR modules to those we report here for hN2 but in addition to sequential unfolding of these, apparently also observes complete unfolding of the HD domain (51). Although these data might support a protein sequence basis for a slight difference in simulated unfolding pathway for the two NRRs, the preliminary nature of the N1-NRR AFM study, its high dependence on the simulation data (50) for interpretation of extension curves, and its use of different methodology to our study prevent a thorough comparison at this stage.
We suggest a mechanism whereby autoinhibition is relieved and S2 cleavage can feasibly occur within hN2-NRR following the removal of the A∶B linker. Interestingly, analysis of S2 site exposure in the hN1-NRR (41) by hydrogen exchange mass spectrometry also deduced that detachment of LNRA and LNRB (upon removal of metal ions) caused sufficient exposure of the S2 site for metalloprotease cleavage, with no requirement for disruption to the structural integrity of the HD domain. The force exerted upon the NRR during the transendocytosis process in vivo is unknown, but, if our proposed mechanism is to hold, it must be sufficient to remove the A∶B linker. Our hN2-NRR AFM data show full unfolding of LNRA and B occurs at forces a little less than 200 pN. Endocytosis has previously been observed to produce uptake forces of around 20–80 pN (at a similar loading rate to that used here) (52). Recently, epsin-dependent clathrin-mediated endocytosis of the mammalian Notch ligand, Delta-like1, has been shown to generate a 10-pN net force on a bead-tethered N-terminal fragment of rat Notch1 (excluding the NRR) (53). Although these measured endocytic force levels may appear low, no experimental single-molecule system has yet mimicked the ligand-induced Notch signaling event between two cells. The tethering of the NRR to a solid substrate in our experimental setup, rather than a membrane (or a movable bead), may indeed inflate recorded forces in AFM experiments compared with those that occur in vivo. It is also the case that other signaling processes are known that entail internalization of a full transmembrane ligand protein by transendocytosis (54), indicative of endocytic force reaching a level sufficient to remove a protein entirely from a membrane. Recorded forces for removal of helices from membranes are in excess of 200 pN (55). Overall, the forces recorded here for LNR unfolding could fit with an endocytic mechanism for forced exposure of the S2 site in Notch, especially since we propose that removal of the A∶B linker is sufficient for cleavage. It is still possible that some local unfolding of the β5 strand may further increase the cleavage propensity and Notch activation, especially if the stability of this region is affected, as is thought to be the case in T-cell acute lymphoblastic leukemia lymphoma causing mutations that occur within the HD domain of human Notch1 (9).
A vital part of our study is that we have been able to show experimentally that injection of metalloproteases during forced unfolding causes hN2-NRR cleavage at the S2 site. The force clamp, 200 pN, was selected based on the forces recorded during the standard AFM unfolding of the hN2-NRR and on the basis that MD simulations predicted that removal of LNRA and LNRB would expose the S2 site. Though AFM is a sensitive approach, it is unfeasible to control it to the level where only LNRA and the A∶B linker are unraveled. The selected force clamp would ensure removal of at least the first two LNRs when metalloprotease is injected; a reasonable compromise. The frequency of hN2-NRR cleavage events observed was significant in the given experimental conditions and directly links force application to exposure and cleavage of the S2 site.
Interestingly, TACE cleaved the S2 cleavage site of hN2-NRR more readily than ADAM10 within all experiments mentioned in this study. Certainly, ADAM10 is more dependent than TACE on incubation at 37 °C, and this is likely the main factor for a lower percentage of cleavage occurring in both the Western blot and AFM experiments (performed at room temperature). However, because recent research has highlighted an essential role for ADAM10 in ligand-dependent activation (14), whereas ADAM10 and TACE have redundant roles in ligand independent activation (14, 56), we cannot completely exclude the possibility that the AFM force-clamp setup may be a less accurate reflection of the natural ligand-dependent forced unfolding process.
All the data presented show that exposure of the S2 site occurs early on the hN2-NRR unfolding pathway at force levels that correlate reasonably well with the adhesion strength between Notch and its ligand (21, 57) and with ligand endocytic force. We propose that the first and most critical force barrier in NRR unfolding (A∶B linker removal) is the mechanosensing event within the Notch extracellular domain. Although A∶B removal is critical, the NRR is actually quite “mechanoresistant” with respect to full unfolding of the individual domains, especially upon metal ion coordination. This mechanoresistance could feasibly enable ready restoration of Notch structure should other factors release the bound Notch ligand. The major contribution of metal ion coordination to NRR mechanoresistance could have physiological significance also. For example, the level of Notch signaling has already been linked to extracellular concentrations of calcium (43), and there is an additional possibility that exposure to a drop in pH such as occurs during endocytosis could affect metal coordination and rate of cleavage at S2. The Notch NRR in the full-length receptor is preceded by EGF-like repeats, which would be predicted to offer considerable mechanical stability also, based on the precedence of β-sheet dominated structures to show the highest mechanical stability (37–39). Future work to test the influence of these preceding EGF-like repeats would, however, be of interest. A further issue to explore is whether cleavage at S1 is required for S2 cleavage, for which contradictory data exist (23, 58, 59), probably due to differences in organism and type of Notch receptor. Here, we show protease accessibility to S2 upon forced unfolding of hN2-NRR that possesses an intact S1 site. This result would concur with the observation that S1-resistant Notch2 retains signaling competency (60). S1-resistant Notch1 has reduced signaling competency, however, and similar forced unfolding work with this receptor may yield different observations. The influence of S1 cleavage (and, indeed, other factors) on mechanical unfolding and S2 exposure would be best tested using a single-molecule experimental setup that tethered the NRR (of different Notch receptor types) to a membrane. Current work is directed toward these technically challenging experiments, ideally with detection of Notch signal transmission to a cell nucleus after force activation. Although questions, therefore, remain concerning Notch mechanotransduction, we have combined experimental and computational modeling approaches to reveal new direct evidence for, and insight into, the molecular mechanism of the process. Further methodological developments will help answer the remaining questions, with potential for therapeutic applications in Notch-related disease, as well as enhance exploration of mechanical force in other signaling pathways.
Materials and Methods
Protein Expression and Purification.
Recombinant NRR was produced as a GST-fusion protein with N-terminal poly-lysine (Lys3) and C-terminal hexahistidine (His6) tags through expression in T7 Express Escherichia coli cells (New England Biolabs). Cells were lysed by addition of lysozyme and three rounds of freeze thaw. Protein was purified from soluble lysate through incubation with glutathione beads (GE Healthcare), cleaving the GST-tag to release the protein. To gain further purity, the protein was denatured and purified further with ion-exchange (Ni2+-His; His-Trap™ HP columns) and size exclusion chromatography (S-200 Superdex) (columns from GE Healthcare). Purified protein was refolded in a redox buffer (containing 5 mM cysteine, 1 mM cystine, and 50 mM CaCl2) before structural characterization was performed to determine correct structural conformation (1D-1H NMR, 2D 1H-NOESY NMR, CD, UV resonance Raman spectroscopy).
Fluorescent Peptide Cleavage.
Quenched peptide (MCA-GSYPLVSVVSE-Dap(DNP)-SLT-NH2; Generon) (10 mM) in 25 mM Tris-HCl, 2.5 μM ZnCl2, pH 9.0 was incubated for 5 min with 0.1 ng/μL TACE (mature soluble extracellular region, R&D Systems) at room temperature or 0.1 ng/μL ADAM10 (R&D Systems) at 37 °C before being excited at 320 nm. Absorption spectra were recorded (320–600 nm) every 5 min to a total of 90 min at either room temperature (TACE) or 37 °C (ADAM10). The absorption peak at 405 nm was used to follow change in activity over time.
Mass Spectrometry of Peptide Cleavage.
The same quenched peptide (0.24 mM) in 25 mM Tris-HCl, 2.5 μM ZnCl2, pH 9.0 was incubated for 24 h with 0.78 μg TACE (mature soluble extracellular region, R&D Systems) at room temperature. The reaction was then purified using a ZipTip (Merck Millipore) eluted into 5 μL 50% Acetonitrile, 0.1% Trifluoroacetic acid. Mass spectrometry analysis was performed on a Bruker Ultraflex II TOF/TOF using 1 μL eluted material in a 1∶1 ratio with α-Cyano-4-hydrozycinnamic acid matrix (10 mg/mL in 50% ethanol, 50% acetonitrile).
Western Blot Cleavage.
Denatured (incubated overnight in 8 M urea) and wild-type recombinant hN2 Lys3-NRR-His6 was diluted 1 in 100 with a cleavage buffer containing zinc ions (25 mM Tris-HCl, 2.5 μM ZnCl2, pH 9.0) and incubated at room temperature for 24 h with either 0.1 ng/μL TACE or 0.1 ng/μL ADAM10. Results were analyzed by Western blot. The blot on Hybond C (GE Healthcare) was blocked with 5% milk powder in TBS+Tween (2 h), incubated with HRP conjugated mouse anti-His6 antibody (Invitrogen) in blocking solution (16 h), washed, and viewed by using ECL Plus Western Blotting kit (Pierce) and exposing to film.
Standard AFM Experiments.
Gold-coated AFM tips were functionalized with Ni2+-NTA by incubating overnight with a 0.01 mM NTA-alkanethiol/0.04 mM EG3-alkanthiol solution (obtained from Prochimia and Nanoscience Instruments, respectively), followed by 1-h incubation with nickel sulphate (Sigma Aldrich). Gold-coated AFM slides were functionalized by incubating overnight with a 0.1 mM NHS-alkanethiol/0.04 mM EG3-alkanthiol solution (both obtained from Nanoscience Instruments), followed by a 1-h incubation with protein solution in PBS, and 1 M ethanolamine (Sigma Aldrich) incubation for 40 min. Force experiments were performed on a Nanoscope V controller in PBS buffer at a loading rate of approximately 1 × 10-7 N/s (spring constant approximately 67 pN/nm; velocity approximately 1,600 nm/s), with a 2-s surface delay to ensure nickel-His coordination (based on previous experiments). Controls were performed without protein present to determine the level of features occurring due to interactions between the functionalized slide and tip. Surface adhesion contacts appearing at the foot of the retraction slope, distinct from unfolding features observed when protein is present, could thus be identified. Attachment controls were also performed using competing ions to disrupt Ni2+-His interactions at the tip.
AFM Experiments with Cleavage.
Functionalization was performed as above; force experiments were performed on the JPK CellHesion200 in PBS at a loading rate of approximately 0.6 × 10-7 N/s (spring constant approximately 67 pN/nm; velocity 850 nm/s). A 2-s surface delay was used, before a force clamp of 200 pN was maintained for 300 s (5 min). At approximately 30 s, 1.32 μL of solution was injected. Solutions injected were buffer (B; 25 mM Tris-HCl+2.5 μM zinc chloride, pH 9.0), ADAM10 (A; 50 ng/μL ADAM10 in buffer), TACE with required ions (T+; 50 ng/μL TACE in buffer), TACE lacking required ions (T-; 50 ng/μL TACE in 25 mM Tris-HCl, pH 9.0), denatured TACE (dT; 50 ng/μL TACE in buffer, following three rounds of freeze thaw to denature (inactive in fluorescent peptide cleavage experiments).
MD Simulations.
MD simulations were performed using GROMACS (61) version 4.5.3 on hN2-NRR (PDB ID code 2OO4) with and without coordinated ions present. Protein termini were protonated, and titratable amino acids were assigned their canonical state at physiological pH. The force field selected was the GROMOS96 53A6 parameter set (62). Short-range interaction cutoff was set to 1.4 nm and long-range electrostatics were calculated with the particle mesh Ewald algorithm (63, 64). Dispersion correction was applied to energy and pressure terms to account for truncation of van der Waals terms. Periodic boundary conditions were applied in all directions.
Protein (coordinates) was placed in a three-dimensional box (dimensions: 10 × 10 × 50 nm) of 100 nM NaCl in simple point charge water (65), including neutralizing counterions. Steepest descent energy minimization was performed followed by a two-step equilibration, with position restraints applied to heavy atoms. Equilibration step one simulated 100 ps under the NVT ensemble (maintaining a constant number of particles, volume, and temperature). Temperature was maintained at 310 K (37 °C) by coupling protein and nonprotein atoms to separate temperature coupling baths [Berendsen weak coupling method (66)]. Equilibration step two simulated 100 ps under the NPT ensemble, maintaining a constant isotropic pressure of 1.0 bar (weak coupling). All position restraints were then removed, except for those on the atoms of the C-terminal residue (Thr1672), which was used as an immobile reference for the pull simulation. For each simulation, the atoms of the N-terminal residue (Cys1425) were pulled along the z axis at a loading rate of approximately 1 N/s (spring constant: 1.66 × 10-9 N/nm; velocity: 1 × 109 nm/s. These simulations used the Nosé–Hoover thermostat (67, 68) and the Parrinello–Rahman barostat (69, 70). Pearson’s linear correlation coefficient was calculated across five of the obtained simulation data sets using MATLAB R2010a.
Supplementary Material
ACKNOWLEDGMENTS.
We thank the late Anne-Marie Buckle for a collaboration that generated the ideas and confidence to initiate this work. We thank Steven Marsden for his assistance in the Bionanotechnology facility in the Faculty of Life Sciences, Andrew Doig for his advice during the course of the project, Kathryn Blount for reading and improving the manuscript, and the GROMACS Users List for their assistance with our queries. This work was supported by the Biotechnology and Biological Sciences Research Council (UK) via a doctoral training award to N.L.S. (Award BB/D526561/1).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 16412 (volume 109, number 41).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205788109/-/DCSupplemental.
References
- 1.Tien AC, Rajan A, Bellen HJ. A Notch updated. J Cell Biol. 2009;184:621–629. doi: 10.1083/jcb.200811141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson ER, Sandberg R, Lendahl U. Notch signalling: Simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signalling: Cell fate control and signal integration in development. Science. 1990;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 4.Bray SJ. Notch signaling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 5.Meier-Stiegen F, et al. Activated Notch1 target genes during embryonic cell differentiation depend on the cellular context and include lineage determinants and inhibitors. PLoS One. 2010;5:e11481. doi: 10.1371/journal.pone.0011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rampal R, Luther KB, Haltiwanger RS. Notch signalling in normal and disease states: Possible therapies related to glycosylation. Curr Mol Med. 2007;7:427–445. doi: 10.2174/156652407780831593. [DOI] [PubMed] [Google Scholar]
- 7.Weng AP, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 8.Louvi A, Arboleda-Velasquez JF, Artavanis-Tsakonas S. CADASIL: A critical look at a Notch disease. Dev Neurosci. 2006;28:5–12. doi: 10.1159/000090748. [DOI] [PubMed] [Google Scholar]
- 9.Aster JC, Blacklow SC, Pear WS. Notch signalling in T-cell lymphoblastic leukaemia/lymphoma and other haematological malignancies. J Pathol. 2011;223:262–273. doi: 10.1002/path.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koch U, Radtke F. Notch signalling in solid tumors. Curr Top Dev Biol. 2010;92:411–455. doi: 10.1016/S0070-2153(10)92013-9. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Irizarry C, et al. Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol Cell Biol. 2004;24:9265–9273. doi: 10.1128/MCB.24.21.9265-9273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon WR, et al. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 13.Brou C, et al. A novel proteolytic cleavage involved in Notch signalling: The role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 14.Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in Notch signalling. Mol Cell Biol. 2009;29:5679–5695. doi: 10.1128/MCB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehon RG, et al. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- 16.Fleming RJ. Structural conservation of Notch receptors and ligands. Semin Cell Dev Biol. 1998;9:599–607. doi: 10.1006/scdb.1998.0260. [DOI] [PubMed] [Google Scholar]
- 17.Gordon WR, et al. Structure of the Notch1-negative regulatory region: Implications for normal activation and pathogenic signalling in T-ALL. Blood. 2009;113:4381–4390. doi: 10.1182/blood-2008-08-174748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebay I, et al. Specific EGF repeats of Notch mediate interactions with Delta and Serrate: Implications for Notch as a multifunctional receptor. Cell. 1991;67:687–699. doi: 10.1016/0092-8674(91)90064-6. [DOI] [PubMed] [Google Scholar]
- 19.Xu A, Lei L, Irvine KD. Regions of Drosophila Notch that contribute to ligand binding and the modulatory influence of Fringe. J Biol Chem. 2005;280:30158–30165. doi: 10.1074/jbc.M505569200. [DOI] [PubMed] [Google Scholar]
- 20.Cordle J, et al. A conserved face of the Jagged/Serrate DSL domain is involved in Notch trans-activation and cis-inhibition. Nat Struct Mol Biol. 2008;15:849–857. doi: 10.1038/nsmb.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahimou F, Mok LP, Bardo B, Wesley C. The adhesion force of Notch with Delta and the rate of Notch signaling. J Cell Biol. 2004;167:1217–1229. doi: 10.1083/jcb.200407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 23.Nichols JT, et al. DSL ligand endocytosis physically dissociates Notch1 heterodimers before activating proteolysis can occur. J Cell Biol. 2007;176:445–458. doi: 10.1083/jcb.200609014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun X, Artavanis-Tsakonas S. Secreted forms of DELTA and SERRATE define antagonists of Notch signaling in Drosophila. Development. 1997;124:3439–3448. doi: 10.1242/dev.124.17.3439. [DOI] [PubMed] [Google Scholar]
- 25.Varnum-Finney B, et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signalling. J Cell Sci. 2000;113:4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 26.Vas V, Szilágyi L, Pálóczi K, Uher F. Soluble Jagged-1 is able to inhibit the function of its multivalent form to induce hematopoietic stem cell self-renewal in a surrogate in vitro assay. J Leukoc Biol. 2004;75:714–720. doi: 10.1189/jlb.1003462. [DOI] [PubMed] [Google Scholar]
- 27.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White CR, Frangos JA. The shear stress of it all: The cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecuit T, Lenne P-F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Kaksonen M, Drubin DG, Oster G. Endocytic vesicle scission by lipid phase boundary forces. Proc Natl Acad Sci USA. 2006;103:10277–10282. doi: 10.1073/pnas.0601045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedland JC, Lee MH, Boettinger D. Mechanically activated integrin switch controls a5b1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 32.Sukharev S, Anishkin A. Mechanosensitive channels: What can we learn from ‘simple’ model systems? Trends Neurosci. 2004;27:345–351. doi: 10.1016/j.tins.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Sawada Y, et al. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA. 1998;95:2515–2519. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrion-Vazquez M, Oberhauser AF, Fernandez JM. Mechanical and chemical unfolding of a single protein: A comparison. Proc Natl Acad Sci USA. 1999;96:3694–3699. doi: 10.1073/pnas.96.7.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steward A, Toca-Herrera JL, Clarke J. Versatile cloning system for construction of multimeric proteins for use in atomic force microscopy. Protein Sci. 2002;11:2179–2183. doi: 10.1110/ps.0212702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rief M, Gautel M, Oesterhelt F, Fernandez JM, Gaub HE. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science. 1997;276:1109–1112. doi: 10.1126/science.276.5315.1109. [DOI] [PubMed] [Google Scholar]
- 38.Oberhauser AF, Badilla-Fernandez C, Fernandez JM. The mechanical hierarchies of fibronectin observed with single-molecule AFM. J Mol Biol. 2002;319:433–447. doi: 10.1016/S0022-2836(02)00306-6. [DOI] [PubMed] [Google Scholar]
- 39.Bullard B, et al. The molecular elasticity of the insect flight muscle proteins projectin and kettin. Proc Natl Acad Sci USA. 2006;103:4451–4456. doi: 10.1073/pnas.0509016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans E, Ritchie K. Strength of a weak bond connecting flexible polymer chains. Biophys J. 1999;76:2439–2447. doi: 10.1016/S0006-3495(99)77399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiyanont K, et al. Evidence for increased exposure of the Notch 1 metalloprotease cleavage site upon conversion to an activated conformation. Structure. 2011;19:546–554. doi: 10.1016/j.str.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rand MD, et al. Calcium depletion dissociates and activates heterodimeric Notch receptors. Mol Cell Biol. 2000;20:1825–1835. doi: 10.1128/mcb.20.5.1825-1835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raya A, et al. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- 44.Maskos K, et al. Crystal structure of the catalytic domain of human tumor necrosis factor-alpha-converting enzyme. Proc Natl Acad Sci USA. 1998;95:3408–3412. doi: 10.1073/pnas.95.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopan R, Ilagan MX. The canonical Notch signalling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Best RB, Li B, Steward A, Daggett V, Clarke J. Can non-mechanical proteins withstand force? Stretching barnase by atomic force microscopy and molecular dynamics simulation. Biophys J. 2001;81:2344–2356. doi: 10.1016/S0006-3495(01)75881-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dietz H, Rief M. Exploring the energy landscape of GFP by single-molecule mechanical experiments. Proc Natl Acad Sci USA. 2004;101:16192–16197. doi: 10.1073/pnas.0404549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West DK, Brockwell DJ, Olmsted PD, Radford SE, Paci E. Mechanical resistance of proteins explained using simple molecular models. Biophys J. 2006;90:287–297. doi: 10.1529/biophysj.105.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sotomayor M, Schulten K. Single-molecule experiments in vitro and in silico. Science. 2007;316:1144–1148. doi: 10.1126/science.1137591. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Zolkiewska A. Force-induced unfolding simulations of the human Notch1 negative regulatory region: Possible roles of the heterodimerization domain in mechanosensing. PLoS One. 2011;6:e22837. doi: 10.1371/journal.pone.0022837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dey A, Szoskiewicz R. Complete noise analysis of a simple force spectroscopy AFM setup and its applications to study nanomechanics of mammalian Notch 1 protein. Nanotechnology. 2012;23:175101. doi: 10.1088/0957-4484/23/17/175101. [DOI] [PubMed] [Google Scholar]
- 52.Shan Y, et al. Recording force events of single quantum-dot endocytosis. Chem Commun. 2011;47:3377–3379. doi: 10.1039/c1cc00040c. [DOI] [PubMed] [Google Scholar]
- 53.Meloty-Kapella L, Shergill B, Kuon J, Botvinick E, Weinmaster G. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev Cell. 2012;22:1299–1312. doi: 10.1016/j.devcel.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cagan RL, Krämer H, Hart AC, Zipursky SL. The bride of sevenless and sevenless interaction: Internalization of a transmembrane ligand. Cell. 1992;69:393–399. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- 55.Oesterhelt F, et al. Unfolding pathways of individual Bacteriorhodopsins. Science. 2000;288:143–146. doi: 10.1126/science.288.5463.143. [DOI] [PubMed] [Google Scholar]
- 56.van Tetering G, et al. Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J Biol Chem. 2009;284:31018–31027. doi: 10.1074/jbc.M109.006775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shergill B, Meloty-Kapella L, Musse AA, Weinmaster G, Botvinick E. Optical tweezers studies on Notch: Single-molecule interaction strength is independent of ligand endocytosis. Dev Cell. 2012;22:1313–1320. doi: 10.1016/j.devcel.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kidd S, Lieber T. Furin cleavage is not a requirement for Drosophila Notch function. Mech Dev. 2002;115:41–51. doi: 10.1016/s0925-4773(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 59.Pratt EB, et al. The cell giveth and the cell taketh away: An overview of Notch pathway activation by endocytic trafficking of ligands and receptors. Acta Histochem. 2011;113:248–255. doi: 10.1016/j.acthis.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon WR, et al. Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS One. 2009;4:e6613. doi: 10.1371/journal.pone.0006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 62.Oostenbrink C, Villa A, Mark AE, Van Gunsteren WF. A biomolecular force field based on the free enthalpy of hydration and salvation: The GROMOS force-field parameter sets 53A5 and 53A6. J Comput Chem. 2004;25:1656–1676. doi: 10.1002/jcc.20090. [DOI] [PubMed] [Google Scholar]
- 63.Darden T, York D, Pedersen L. Particle mesh Ewald: An n•log(n) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 64.Essmann U, et al. A smooth particle mesh Ewald method. J Chem Phys. 1995;103:8577–8593. [Google Scholar]
- 65.Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J. In: Intermolecular Forces. Pullman B, editor. Dordrecht, The Netherlands: Reidel; 1981. pp. 331–342. [Google Scholar]
- 66.Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular-dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 67.Nosé SA. A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys. 1984;81:511–519. [Google Scholar]
- 68.Hoover WG. Canonical dynamics: Equilibrium phase-space distributions. Phys Rev A. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 69.Nosé S, Klein ML. Constant pressure molecular dynamics for molecular systems. Mol Phys. 1983;50:1055–1076. [Google Scholar]
- 70.Parrinello M, Rahman A. Polymorphic transitions in single crystals: A new molecular dynamics method. J Appl Phys. 1981;52:7182–7190. [Google Scholar]