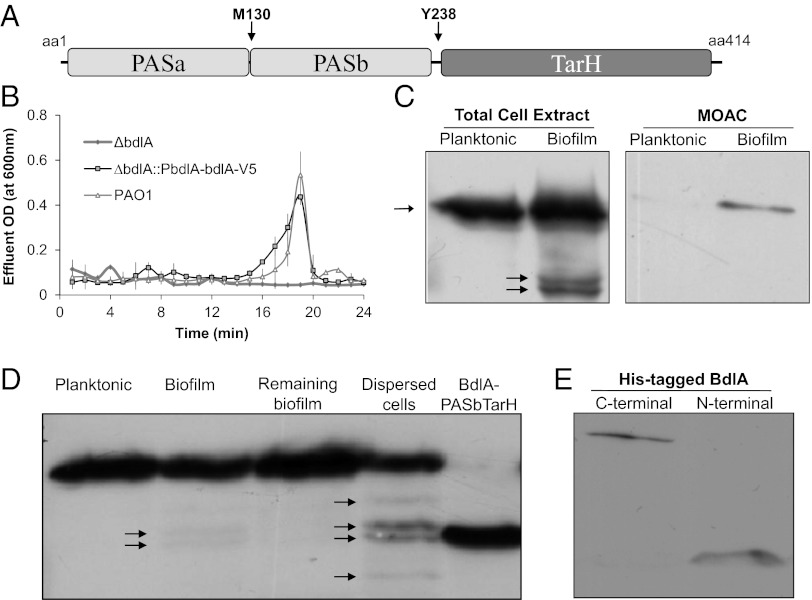
Fig. 1.
Posttranslational modification and nonprocessive proteolysis of BdlA are growth-mode–dependent. (A) Domain structure of BdlA. MCP/TarH, homologue of Tar (Taxis toward aspartate and related amino acids) chemoreceptor domain; PAS, Per Arnt Sim sensory domain. Arrows indicate the position of the methionine-130 (M130) putative cleavage site and the tyrosine-238 (Y238) potential phosphorylation site. (B) cis-encoded (∆bdlA/pbdlA-bdlA-V5/His) C-terminally V5/His-tagged BdlA restores biofilm dispersion phenotype of ∆bdlA to wild-type levels. (C) Detection of cis-encoded V5/His-tagged BdlA in total cell extracts or MOAC-enriched phosphoproteomes of planktonic and biofilm P. aeruginosa cells. (D) BdlA processing is increased in dispersed cells, with the cleavage product being similar in size to BdlA lacking the PASa domain (BdlA-PASbTar). (E) The N-terminal BdlA-PASa remains detectable in cells as indicated using N- and C-terminally His-tagged BdlA proteins obtained from PAO1/pJN-His-bdlA and PAO1/pJN-bdlA-V5/His biofilms. Arrows indicate intact BdlA and its degradation products.