Abstract
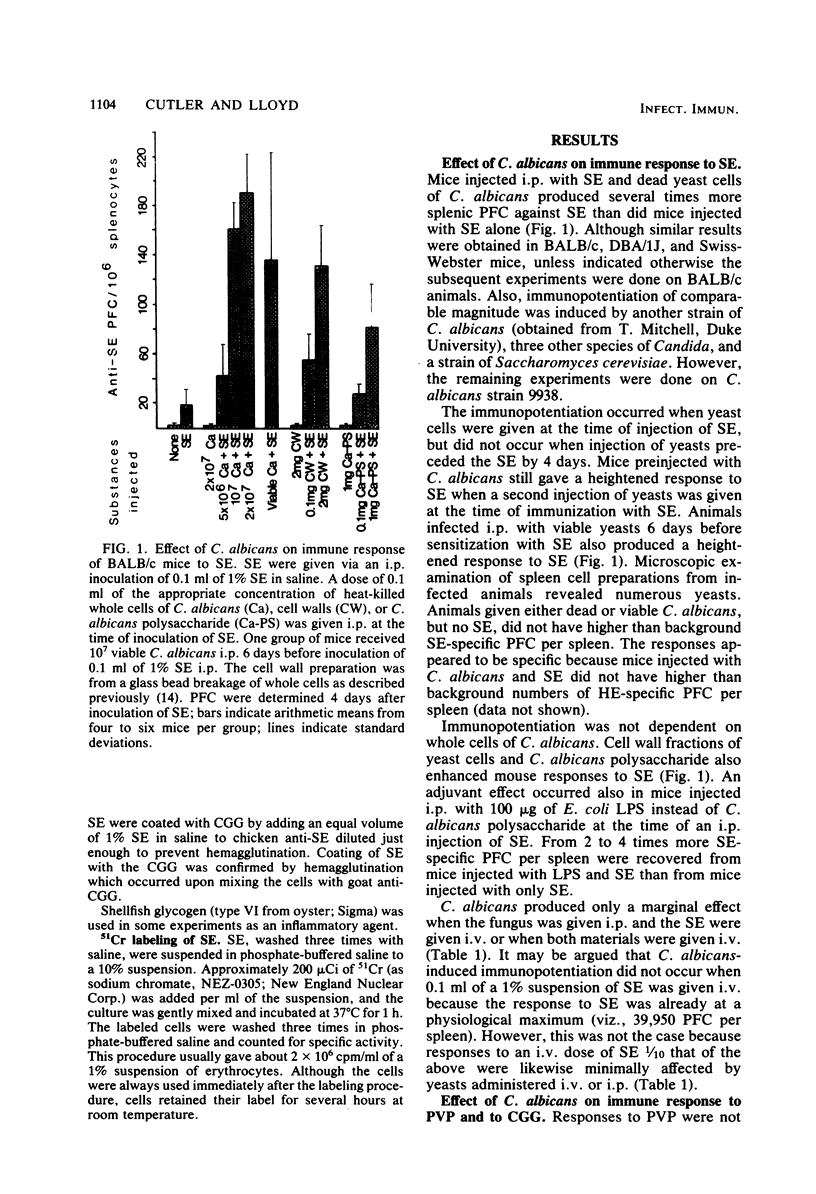
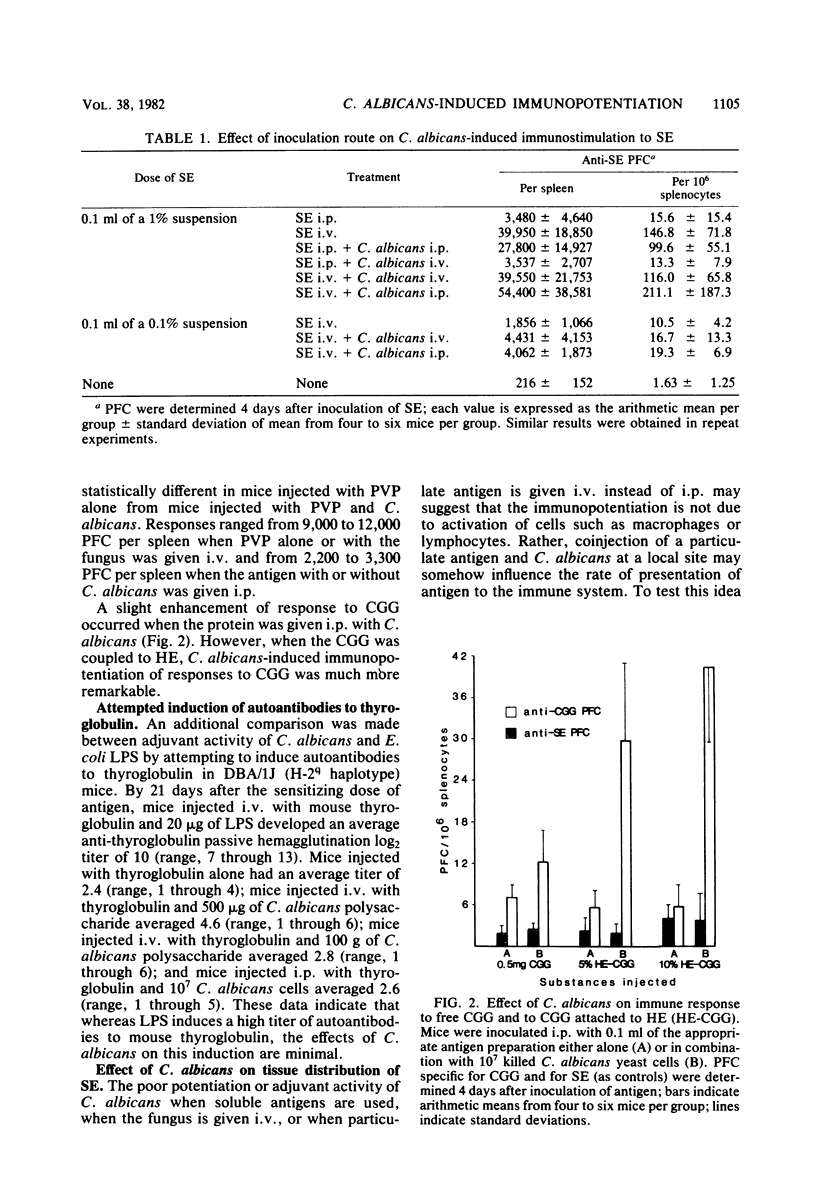
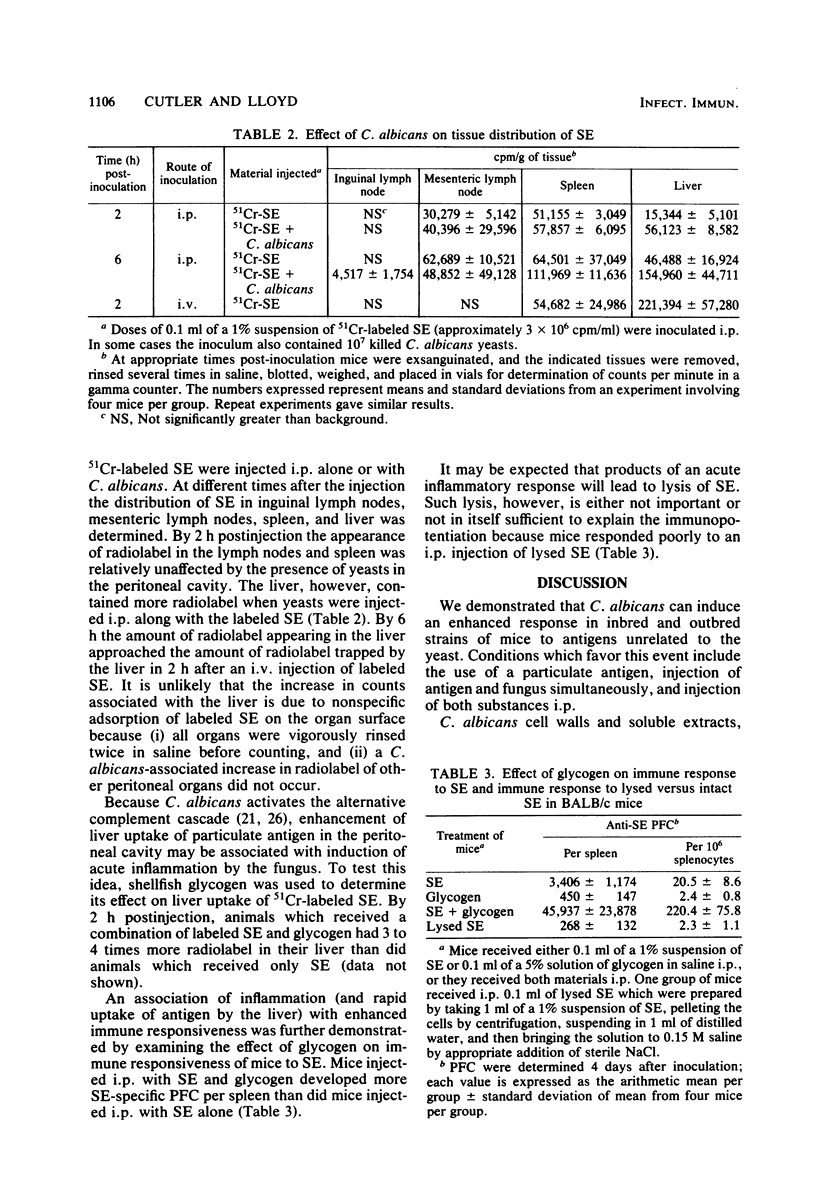
Candida albicans may immunopotentiate antibody responses in mice to antigens unrelated to the fungus. This effect occurred best with cell-associated, rather than soluble, antigens. When dead yeasts, cell walls, or a water-soluble candidal polysaccharide were used, immunopotentiation was most dramatic when the antigen and fungal materials were given concomitantly via an intraperitoneal injection. However, mice infected with viable yeasts several days before antigen administration also developed heightened responses to the antigen. The mechanism of the C. albicans-induced adjuvanticity was not defined, but the effect seemed to correlate with induction of inflammation. The presence of C. albicans or other inflammatory agents in the peritoneal cavity caused a more rapid uptake of particulate antigen by the liver. The relationship between this event and immunopotentiation is not known. These studies demonstrate that C. albicans may have profound effects on host immune responses. Because immunological aberrations are commonly found in patients with candidiasis it may be important to determine whether some of these aberrations result from, rather than precede candidiasis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Cutler J. E., Friedman L., Milner K. C. Biological and chemical characterization of toxic substances from Candida albicans. Infect Immun. 1972 Oct;6(4):616–627. doi: 10.1128/iai.6.4.616-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler J. E., Poor A. H. Effect of mouse phagocytes on Candida albicans in in vivo chambers. Infect Immun. 1981 Mar;31(3):1110–1116. doi: 10.1128/iai.31.3.1110-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Luzio N. R., McNamee R., Browder W. I., Williams D. Glucan:inhibition of tumor growth and enhancement of survival in four syngeneic murine tumor models. Cancer Treat Rep. 1978 Nov;62(11):1857–1866. [PubMed] [Google Scholar]
- Diamantstein T., Keppler W., Blitstein-Willinger E. Suppression of the primary immune response in vivo to sheep red blood cells by B-cell mitogens. Immunology. 1976 Mar;30(3):401–407. [PMC free article] [PubMed] [Google Scholar]
- Dwyer J. M. Chronic mucocutaneous candidiasis. Annu Rev Med. 1981;32:491–497. doi: 10.1146/annurev.me.32.020181.002423. [DOI] [PubMed] [Google Scholar]
- Esquivel P. S., Rose N. R., Kong Y. C. Induction of autoimmunity in good and poor responder mice with mouse thyroglobulin and lipopolysaccharide. J Exp Med. 1977 May 1;145(5):1250–1263. doi: 10.1084/jem.145.5.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost P., Lance E. M. On the mechanism of action of adjuvants. Immunology. 1978 Jul;35(1):63–68. [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Rich R. R., Bennett J. E. Chronic mucocutaneous candidiasis: model-building in cellular immunity. Ann Intern Med. 1971 Jun;74(6):955–978. doi: 10.7326/0003-4819-74-6-955. [DOI] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Narita T., Shimono T., Stewart-Tull D. E. Immunoadjuvant activities of fungal cell walls. Biken J. 1975 Jun;18(2):135–138. [PubMed] [Google Scholar]
- Lake J. P., Reed N. D. Regulation of the immune response to polyvinylpyrrolidone:effect of antilymphocyte serum on the response of normal and nude mice. Cell Immunol. 1976 Feb;21(2):364–370. doi: 10.1016/0008-8749(76)90065-4. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiba H., Matsunaga K., Gojobori M. Effect of immunochemotherapy with OK-432 and yeast cell wall on the activities of peritoneal macrophages of mice. Gan. 1979 Oct;70(5):687–692. [PubMed] [Google Scholar]
- Miller J. F., Warner N. L. The immune response of normal, irradiated and thymectomized mice to fowl immunoglobulin G as detected by a hemolytic plaque technique. Int Arch Allergy Appl Immunol. 1971;40(1):59–71. doi: 10.1159/000230395. [DOI] [PubMed] [Google Scholar]
- Miller M. E., Nilsson U. R. A major role of the fifth component of complement (C5) in the opsonization of yeast particles. Partial dichotomy of function and immunochemical measurement. Clin Immunol Immunopathol. 1974 Jan;2(2):246–255. doi: 10.1016/0090-1229(74)90042-7. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Cutler J. E. In vitro studies of the interaction of murine phagocytic cells with Candida albicans. J Reticuloendothel Soc. 1981 Jan;29(1):23–34. [PubMed] [Google Scholar]
- Mourad S., Friedman L. Passive immunization of mice against Candida albicans. Sabouraudia. 1968 Feb;6(2):103–105. [PubMed] [Google Scholar]
- Nadler P. I., Klingenstein R. J., Richman L. K., Ahmann G. B. The murine Kupffer cell. II. Accessory cell function in in vitro primary antibody responses, mitogen-induced proliferation, and stimulation of mixed lymphocyte responses. J Immunol. 1980 Dec;125(6):2521–2525. [PubMed] [Google Scholar]
- Pearsall N. N., Adams B. L., Bunni R. Immunologic responses to Candida albicans. III. Effects of passive transfer of lymphoid cells or serum on murine candidiasis. J Immunol. 1978 Apr;120(4):1176–1180. [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Human lymphocyte-activating properties of a purified polysaccharide from Candida albicans: B and T cell cooperation in the mitogenic response. J Immunol. 1980 Nov;125(5):2082–2088. [PubMed] [Google Scholar]
- Provost T. T., Garrettson L. K., Zeschke R. H., Rose N. R., Tomasi T. B., Jr Combined immune deficiency, autoantibody formation, and mucocutaneous candidiasis. Clin Immunol Immunopathol. 1973 Jul;1(4):429–445. doi: 10.1016/0090-1229(73)90001-9. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Wuepper K. D. Activation of the alternative (properdin) pathway of complement by Candida albicans and related species. J Invest Dermatol. 1976 Dec;67(6):700–703. doi: 10.1111/1523-1747.ep12598581. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Kastello M. D., Harrington D. G., Crabbs C. L., Peters C. J., Jemski J. V., Scott G. H., Di Luzio N. R. Glucan-induced enhancement of host resistance to selected infectious diseases. Infect Immun. 1980 Oct;30(1):51–57. doi: 10.1128/iai.30.1.51-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- Rogoff T. M., Lipsky P. E. Role of the Kupffer cells in local and systemic immune responses. Gastroenterology. 1981 Apr;80(4):854–860. [PubMed] [Google Scholar]
- Saltarelli C. G., Coppola C. P. Inhibitory effects of Candida albicans extracellular polysaccharides on mouse sarcoma 180. J Surg Oncol. 1980;15(1):99–106. doi: 10.1002/jso.2930150115. [DOI] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Phillips J. K., Mergenhagen S. E. Biological activity of complement in vivo. Role of C5 in the accumulation of polymorphonuclear leukocytes in inflammatory exudates. J Exp Med. 1971 Nov 1;134(5):1131–1143. doi: 10.1084/jem.134.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K., Nomoto K., Matsumoto T., Miyake T., Himeno K. Chronic mucocutaneous candidiasis accompanied by enhanced antibody production. Clin Exp Immunol. 1976 Sep;25(3):497–500. [PMC free article] [PubMed] [Google Scholar]
- Valdimarsson H., Higgs J. M., Wells R. S., Yamamura M., Hobbs J. R., Holt P. J. Immune abnormalities associated with chronic mucocutaneous candidiasis. Cell Immunol. 1973 Mar;6(3):348–361. doi: 10.1016/0008-8749(73)90035-x. [DOI] [PubMed] [Google Scholar]


