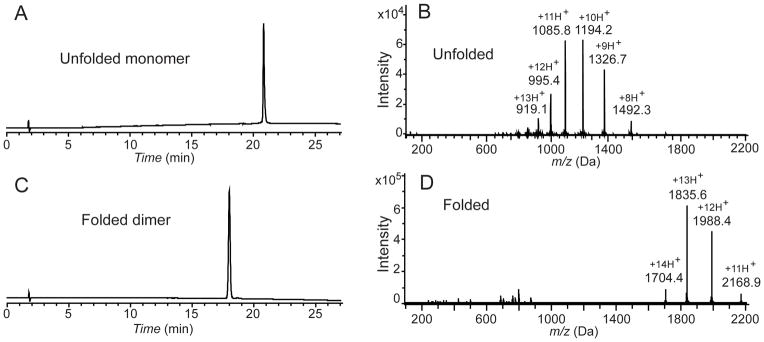
Figure 3.
HPLC and mass spectrometry characterization of synthetic VEGF. (A) Analytical HPLC profile (λ = 214 nm) of the purified unfolded 102 amino acid polypeptide [Gly1-Asp102-COOH]; (B) LC-MS of purified unfolded 102 amino acid polypeptide [Gly1-Asp102-COOH]; observed mass: 11932.2±0.7 Da (average of the four most abundant charge states), calculated mass: 11932.54 Da (average isotope); (C) Analytical HPLC profile (λ = 214 nm) of the purified synthetic VEGF protein. Folded VEGF showed a 2.8 min earlier retention time shift compared to the reduced polypeptide; (D) Direct infusion electrospray ionization MS of the synthetic VEGF protein: observed mass: 23848.7±1 Da (mean of the three most abundant charge states), calculated mass: 23849.1 Da (average isotope composition). A decrease of 15.7 Da from twice the monomer mass confirmed the formation of eight disulfides. Note the narrow distribution of the charge states in the folded VEGF dimer compared with the unfolded monomer. Analytical HPLC was performed using a linear gradient (10̃54%) of buffer B in buffer A over 22 min (buffer A = 0.1% trifluoroacetic acid (TFA) in water; buffer B = 0.08% TFA in acetonitrile) on a C-3 (Agilent), 4.6 ×150 mm column at 40 °C (flow rate = 1 mL/min).