Figure 1.
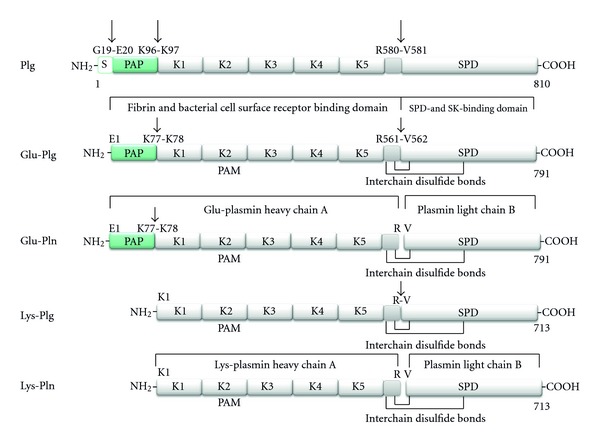
Structural domains of human plasmin(ogen) forms. Human plasminogen is synthesised as an 810 amino acid protein. The 19 amino acid residue signal sequence is removed resulting in the circulating mature form (791 amino acids, ~90,000 kDa) known as Glu-plasminogen (Glu-plg) as it contains an N-terminal glutamic acid. Glu-plg contains a hairpin-loop structure called the PAN domain encompassing the preactivation peptide (PAP), followed by 5 homologous kringle domains (K1–K5) containing three intradomain disulfide bridges, followed by a peptidase S1 domain (SPD). The preactivation peptide is generated by plasmin cleavage giving rise to Lys-plg (713 amino acids, ~80,000 kDa). The conversion of Glu-plg or Lys-plg to their respective plasmin forms occurs by hydrolysis of the Arg-Val peptide bond shown by either uPA or tPA, yielding chain A and the smaller chain B, which remain covalently associated by interchain disulfide bonds. Kringles 1, 2, 4, and 5 contain lysine-binding sites (LBS) with affinity for free lysine and lysine-like compounds such as ω-aminocarboxylic ligands in the following order of binding affinity K1 > K4 > K5 > K2 [8]. Kringle 3 shows no detectable binding to Lys or Lys-like compounds [9], related to a sequence variation in its LBS. Glu-plg thus binds to various lysine-containing proteins via Kringles 1, 2, 4, and 5. Streptokinase (SK) and staphylokinase (not shown) bind in a 1 : 1 complex with the SPD to generate an activator complex. Not shown: Mini plasminogen (K5 plus the SPD) can also be generated by stromelysin-19 cleavage of the Pro466-Val467 bond of Plg. Sequence data are derived from UniProt (swiss-prot entry P00747). Plasminogen (EC = 3.4.21.7) (http://www.uniprot.org/uniprot/P00747).
