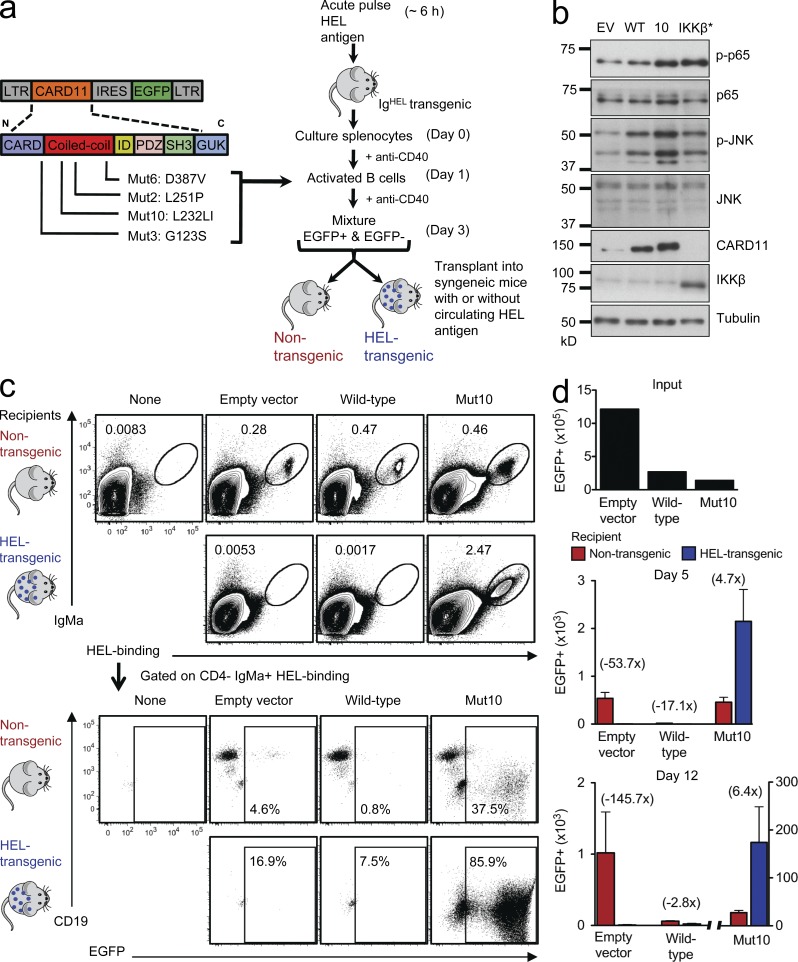
Figure 1.
A potent CARD11 mutation blocks self-antigen–induced B cell death and promotes extensive proliferation in vivo. (a) Experimental strategy to examine the consequences of acquiring different CARD11 coiled-coil domain mutations in normal antigen-activated B cells. (b) Immunoblot for phospho/total p65 and phospho/total JNK in cell lysates from EV, WT, Mut10, and constitutively active IKK-β (IKK-β*)–expressing B cells. Cells cultured in media without anti-CD40 for 24 h before EGFP+ cells were sorted. (c) Antigen-specific B cells transduced with the indicated vectors were transplanted into Rag1-deficient recipient mice that were either nontransgenic, in which the B cells lacked antigen or CD40 stimuli, or were HEL transgenic, in which the transplanted B cells were continuously stimulated by circulating self-antigen. Donor cells in the spleen of recipient mice were analyzed by flow cytometry 5 and 12 d after transplantation. In the top panels, the transplanted B cells were detected and enumerated as a percentage of spleen cells by their binding of HEL antigen and staining for IgMa allotype. The bottom panels are gated on these transplanted B cells and show the percentage that are EGFP+ and their expression of CD19. (d) The top panel shows the number of EGFP+ B cells that were transplanted into each mouse (input). Bottom panels show mean number ± SEM of EGFP+ B cells in the spleen of nontransgenic or HEL transgenic recipient mice on days 5 and 12 (n = 3–6 recipient mice per group and time point; one way analysis of variance [ANOVA]: P = 0.0011 and P = 0.0014, respectively). Data are representative of four independent experiments.