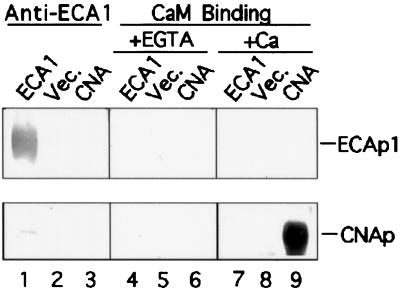
Figure 9.
ECA1 did not bind calmodulin. Membranes were isolated from cells transformed with either vector alone (Vec.) or ECA1. Vesicle protein (5 μg) from the 25%/45% Suc interface was analyzed by SDS-PAGE, blotted onto an Immobilon-P membrane, and probed with an antibody against ECA1 (lanes 1–3). Another blot was probed with biotinylated calmodulin in the absence (+1 mm EGTA, lanes 4–6) or presence of (+Ca2+, lanes 7–9) 0.5 mm Ca2+. The bovine calcineurin A subunit (CNA; 0.2 μg protein/lane) is a calmodulin-binding protein.