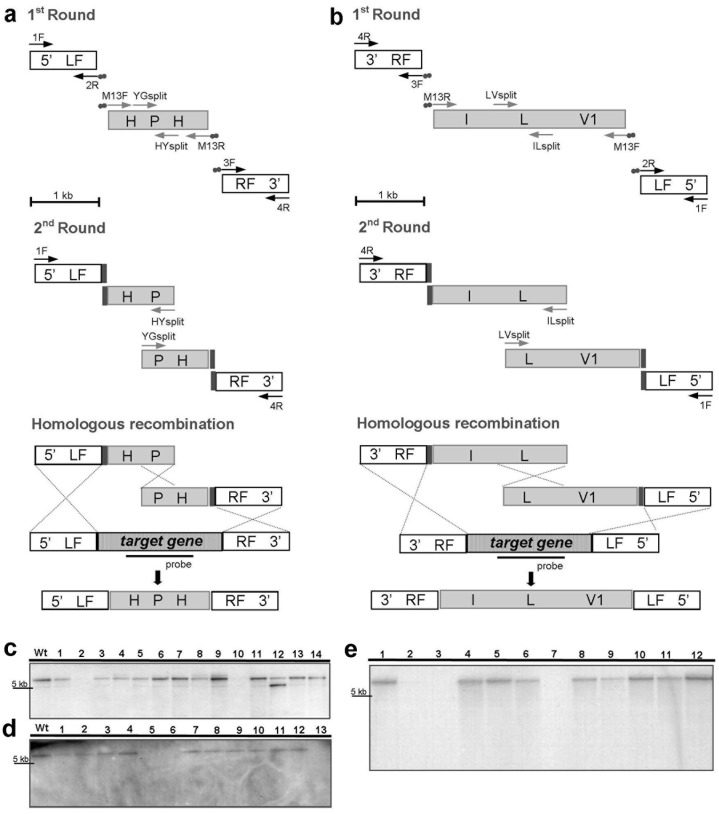
Figure 1. Targeted gene deletion of the N-acetyl-ß-D-glucosaminidase-encoding genes nag1 and nag2 by using the split-marker strategy.
Schematic representations of vector construction are shown in (a) for the single knockout strains, and (b) for the double knockout strains (a) Primers 1F/2R and 3F/4R amplify 1kb target gene flanking sequences. Primers 2R and 3F are hybridised once the 5′ ends complement the M13F and M13R sequences, respectively. For nag1, the primer pairs Chito2_50.1/Chito2_M13F and Chito2_M13R/Chito2_30.1 were used and, for nag2, the primer pairs HEX1_Fw/HEX1.M13F_Rev and HEX2.M13R_Fw/HEX2_Rev were used. Primer pairs M13F/HYsplit and M13R/YGsplit amplify the ‘HP’ and ‘PH’ marker fragments, respectively. Two separate PCR reactions (1F/HYsplit) and (YGsplit/4R) fuse the flanking sequences to the 5′ ‘HP’ or 3′ ‘PH’ fragments of the hygromycin resistance gene hph. Similar steps were used for double knockout mutant generation (b), using a nag2 deletion mutant for transformation with a second selectable marker ilv1 bestowing resistance to sulfonylurea. In this case, the primer pairs used in the first round PCR reactions were Chito2_M13R/Chito2_30.1 and Chito2_50.1/Chito2_M13F for amplification of the 1 kb nag1 flanking sequences and ILsplit/M13R and M13F/LVsplit for amplification of the ‘IL’ and ‘LV1’ fragments, respectively. Second round PCR reactions were performed with the primer pairs Chito2_30.1/ILsplit and LVsplit/Chito2_50.1. Primer sequences are shown in Table 1. (c), Putative ?nag2 transformants. The lane indicated by Wt consists of wild type Tv.223 DNA and lanes 1–14 contain DNA of putative ?nag2 knockouts. The ?Tvnag2 mutant in lane 10 (confirmed by the absence of a band) was selected for nag2 loss-of-function studies and for the generation of ?Tvnag1?Tvnag2 mutants. (d) Putative ?nag1 transformants. The lane indicated by Wt consists of wild type Tv.223 DNA and lanes 1–13 contain DNA of putative ?nag1 knockouts. The ?Tvnag1 mutant in lane 5 (confirmed by the absence of a band) was selected for nag1 loss-of-function studies. (e) Putative ?Tvnag1?Tvnag2 transformants. Lane 1 contains DNA of the ?Tvnag2 mutant (lane 10 in (c)) used for the generation of the double mutants. Lane 2 contains DNA of the ?Tvnag1 mutant from lane 5 in (d). Lanes 3–12 contain DNA of putative double mutants. The double mutant, ?Tvnag1?Tvnag2, in lane 3 was selected for loss-of-function studies.