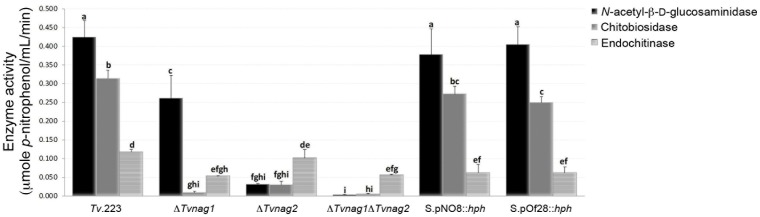
Figure 4. Detection of chitinolytic activities in culture filtrates of the T. virens strains.
N-acetyl-ß-D-glucosaminidase, chitobiosidase and endochitinase activities of the wild type strain Tv.223 and the five chitinase-deficient mutants ?Tvnag1, ?Tvnag2, ?Tvnag1?Tvnag2, S.pNO8::hph, and S.pOf28::hph were determined by enzymatic hydrolysis of the chitinase-specific substrates 4-Nitrophenyl N-acetyl-ß-D-glucosaminide, 4-Nitrophenyl N,N’-diacetyl-ß-D-chitobioside and 4-Nitrophenyl ß-D-N,N’,N’’-triacetylchitotriose, respectively. Substrate hydrolysis releases p-nitrophenol which, upon ionization in basic pH, can be measured colorimetrically at 405 nm. Protein concentrations of the extracts were adjusted to 20 µg.mL−1 prior to enzyme assays. Histograms are the means of three replicate values with standard deviations. One unit of the activity releases 1.0 µmole of p-nitrophenol from the appropriate substrate per minute at pH 4.8 and 37°C. Letters denote the results of a t-test for comparison of means. Bars with different letter(s) are significantly different at 95% confidence level.