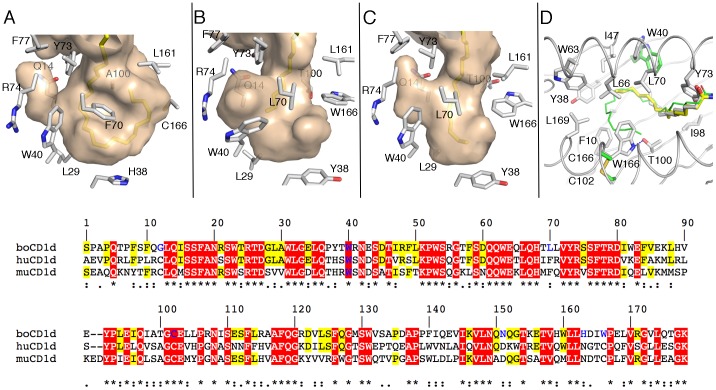
Figure 3. Architecture of the A’ pocket of boCD1d.
. The A’ pocket of human CD1d with bound C16∶0-αGalCer (A), boCD1d with bound C12∶0-di-sufatide (B) and C16∶0-αGalCer (C) is shown as a semi-transparent molecular surface colored in mauve. CD1d residues that influence the shape of the groove are indicated as grey sticks. (D) Superposition of the A’ pocket of boCD1d and huCD1d, with C12∶0-di-sufatide (yellow), C16∶0-αGalCer (grey) and C26∶0-αGalCer (green, from PDB ID 1ZT4) shown. While Trp40 (W40) adopts different orientations in both boCD1d structures, which influences the shape of the small side pocket, Trp166 (W166) blocks the second half of the A’ pocket and restricts the ligand size to 16–18 carbons. Trp166 is a cysteine in both human and mouse CD1d (C102-C166 in green/yellow) that is involved in a disulfide bond with Cys102 located on the β-sheet (C). Sequence alignment of the binding groove forming residues of bovine (bo), human (hu) and murine (mu) CD1d (bottom panel). Red boxed residues are conserved in all three species, while yellow boxed residues are conserved between boCD1d and either human or mouse CD1d. Residues that represent features that are not conserved in boCD1d are colored blue (e.g. A’ pole; G12, L70, C102-C166 disulfide bond, N151 which is important for αGalCer headgroup binding and both Trp40 and Trp166 that influcne the shape of the groove).