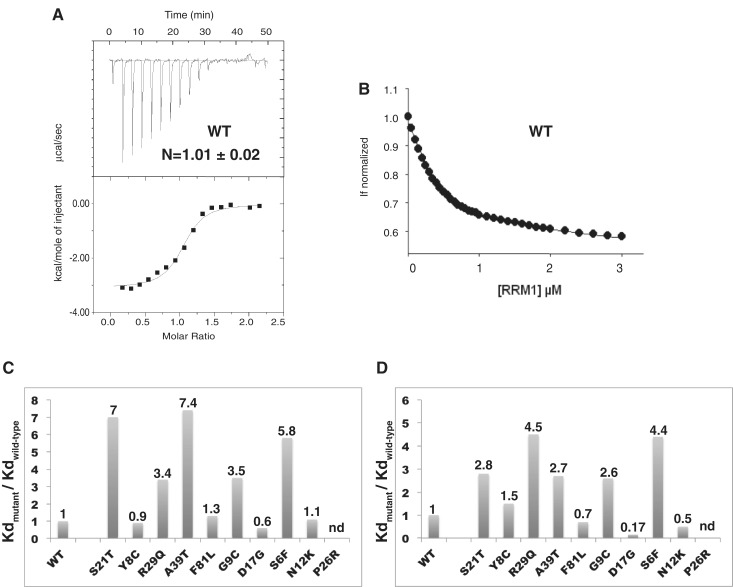
Figure 5.
DNA binding at 25°C. (A) ITC thermodynamic profile obtained for the wild-type protein (15 µM) titrated by DNA (150 µM). The upper isotherm indicates the DNA binding raw data. The lower curve is obtained after integration of individual heat flow signals as function of the DNA/protein molar ratio in the calorimeter cell. (B) Fluorescence titration of W36 (excitation at 295 nm and emission at 324 nm) as a function of increased DNA concentrations, for the wild-type protein (0.5 µM). (C) Histogram of the relative DNA-binding affinities of the mutant proteins determined by ITC. The values reported above the bars indicate the ratios Kdmutant/Kdwild-type. (D) Histogram of the relative DNA-binding affinities of the mutant proteins determined by fluorescence. The proteins are ordered as in Figure 2.