Abstract
Environmental pathogens – organisms that survive in the outside environment but maintain the capacity to cause disease in mammals – navigate the challenges of life in habitats that range from water and soil to the cytosol of host cells. The bacterium Listeria monocytogenes has served for decades as a model organism for studies of host–pathogen interactions and for fundamental paradigms of cell biology. This ubiquitous saprophy te has recently become a model for understanding how an environmental bacterium switches to life within human cells. This review describes how L. monocytogenes balances life in disparate environments with the help of a critical virulence regulator known as PrfA. Understanding L. monocytogenes survival strategies is important for gaining insight into how environmental microbes become pathogens.
Keywords: environmental pathogen, GASP, intracellular pathogen, PrfA
“All living things contain a measure of madness that moves them in strange, sometimes inexplicable ways. This madness can be saving; it is part and parcel of the ability to adapt. Without it, no species would survive.”
– Yann Martel
Whereas obligate human and animal bacterial pathogens can, in general, count upon the warmth and relative stability of their chosen environmental replication niche, environmental bacteria that harbor the ability to replicate both within mammals as well as within the outside environment must maintain a broad array of survival skills to manage life under these disparate conditions. Adaptation to wide ranges of temperature conditions, available nutrients and stresses encountered through physical conditions as well as those resulting from host immunological responses requires an ability to sense and rapidly adapt to new and unfamiliar territories. Examples of survival strategies adopted by environmental bacterial pathogens have been described for several water-borne pathogens, including Vibrio cholerae and Legionella pneumophila [1,2]. V. cholerae makes use of multi-functional gene products such as the chitin-binding protein GbpA that promotes colonization of chitinous exoskeletons of plankton, as well as binding to mucin within the mammalian intestine [3]. L. pneumophila has evolved survival strategies for life within amoebae that contribute to the bacterium’s ability to survive encounters with mammalian macrophages [1]. A number of soil pathogens, such as several Clostridium species and Bacillus anthracis, survive in outside environments via the formation of resistant and long-lasting spores that germinate as conditions become favorable.
The soil-dwelling and food-borne bacterial pathogen Listeria monocytogenes appears to have developed a different set of survival skills for the acquisition of what it needs for replication and survival. This non-spore-forming, Gram-positive bacterium is widespread in nature, where it is thought to live off of decaying plant matter as a saprophyte (Figure 1) [4–6]. L. monocytogenes does not form spores but is capable of adapting to large shifts in environmental temperature, salt concentrations, nutrients and pH [7]. This resilience provides a means for L. monocytogenes to contaminate and proliferate within food supplies despite the use of common preservation methods that serve to quickly eradicate or limit the replication of other harmful microorganisms (Figure 1) [8–10]. As a result, thousands of cases of food-borne illnesses and death, as well as some of the most expensive food recalls in US history, have been linked to L. monocytogenes-tainted food products [10–14]. Here, we describe the survival strategies employed by the soil-dwelling, food-borne nutrient-thief and mammalian pathogen L. monocytogenes to optimize bacterial fitness both inside and outside of host cells. This review will summarize recent findings regarding how the central regulator of L. monocytogenes virulence gene expression, PrfA, helps to coordinate the balance between bacterial life as a saprophyte versus that as an intracellular parasite. For more detailed descriptions of individual L. monocytogenes virulence factors that contribute to life specifically within host cells, readers are referred to several excellent recent reviews [15–17].
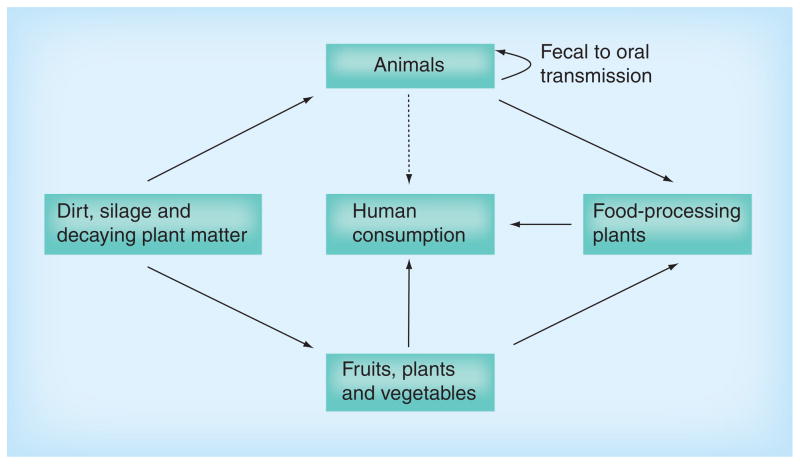
Figure 1. The varied habitats of Listeria monocytogenes.
The bacterium Listeria monocytogenes survives and replicates within diverse environments, ranging from ground water and soil to the cytosol of infected mammalian cells. L. monocytogenes is thought to live as a saprophyte in the outside environment and it has been isolated from soil, decaying plant matter, sewage, silage and water. Animals ingesting L. monocytogenes may become infected and/or may shed the bacterium in feces, facilitating transmission via oral–fecal routes. Food-borne outbreaks of L. monocytogenes have been associated with contaminated fruit and vegetables, and from bacterial contamination of food produced within food-processing plants.
L. monocytogenes as an unwelcome guest at the mammalian cell dinner table
With respect to the pathogenic lifestyle of L. monocytogenes, disease resulting from the infiltration of the bacterium into a mammalian host can take a variety of forms. In healthy persons, exposure to L. monocytogenes-contaminated food products usually results in a self-limiting and mild gastroenteritis [18,19]. By contrast, in individuals who are immunocompromised such as the elderly, chemotherapy or transplant patients and pregnant women [20], serious L. monocytogenes systemic infections can manifest as meningitis, encephalitis and bacteremia, resulting in death or fetal infection and stillbirth in the case of pregnant women. While the disease listeriosis is not as commonly reported as infections resulting from other food-borne pathogens, it does have one of the highest case fatality rates [20].
L. monocytogenes can infect a wide variety of host species and cell types, with the primary route of infection of humans occurring through the consumption of contaminated food products. Once ingested, the bacteria translocate across the intestinal epithelium to obtain access to underlying tissues [19,21]. Having crossed the intestinal barrier, the bacteria enter into the bloodstream and are taken up by resident macrophages within the liver or by dendritic cells within the spleen, where they are either subsequently cleared by an effective host immune response or disseminate onto other organs [22]. While best known for targeting the CNS and the placenta [18], invasive L. monocytogenes can also target other organs such as the heart [23,24], bone marrow [25] and the gall bladder [26].
Multifunctional bacterial gene products contribute to L. monocytogenes survival within mammalian cells
The move from soil to cytosol requires a number of L. monocytogenes factors that promote bacterial invasion, phagosomal escape, the theft of host cell nutrients and spread to adjacent cells [15]. Bacterial gene products contributing to many key aspects of host infection have been identified and discussed in recent reviews [15–17], and new factors and associated functions continue to emerge. Entry of the bacterium into professional phagocytes occurs via phagocytosis, whereas entry into nonprofessional phagocytic cells is mediated by the expression of surface proteins that promote bacterial attachment and invasion [15], with well-characterized examples being the internalins InlA and InlB [27,28]. Following cell uptake, L. monocytogenes escapes from host cell vacuoles via the secretion of the pore forming cytolysin LLO and two phospholipases, PI-PLC encoded by plcA and a broad-range PLC (PC-PLC) encoded by plcB [29–32]. Once L. monocytogenes resides within the cytosol, the bacterium adapts metabolically to use host-provided nutrients by shifting from glycolysis to the oxidative pentose phosphate pathway [33] and by scavenging phoshporylated sugars, glycerol, lipoic acid, branched chain amino acids and peptides [34–39]. Bacterial spread to adjacent cells occurs using actin polymerization as a motile force, a process that is dependent upon expression of the surface protein ActA [40]. The breaking and entry of L. monocytogenes into adjacent cells is further facilitated through the relief of cortical tension by the internalin InlC [41]. Escape from the double-membrane vacuoles formed as a result of L. monocytogenes cell-to-cell spread is dependent upon the activities of LLO, PC-PLC and PI-PLC [30–32]. In addition to the virulence factors just described, a number of other gene products that contribute to L. monocytogenes life within host cells have been identified, many of which may have multiple functional roles [42]. L. monocytogenes has thus clearly developed a complex and multifunctional virulence factor arsenal to stake out its replication domain within mammalian host cells.
Coordinating virulence factor expression within the host, or how L. monocytogenes increases the odds for intracellular survival
Like every successful gambler, L. monocytogenes does not show its cards until it is seated at its eukaryotic dinner table. The expression of a number of bacterial virulence factors appears to be coordinated with bacterial entry into the host or into the cell cytosol. A number of studies have focused on the identification of bacterial genes expressed within tissue culture cells, within blood or within infected animals as a means of identifying bacterial gene products that contribute to intracellular survival [6,34,35]. Microarray analyses of bacterial transcripts induced during L. monocytogenes infection of tissue culture cells revealed that approximately 20% of bacterial genes were differentially expressed, including genes with products having established roles in bacterial virulence [34,35]. Genes with increased expression in cytosolic bacteria included those involved in general stress responses, cell division, modification of the cell wall and in the use of carbon sources such as glycerol and phosphorylated sugars, implicating the pentose phosphate pathway as the major metabolic pathway for carbon utilization within the host cell [34,43]. Transcriptional profiling of L. monocytogenes genes expressed during in vivo growth in mouse spleens also indicated that approximately 20% of bacterial genes were differentially expressed [44]. Similar to the findings reported for bacteria grown within tissue culture cells, genes induced in vivo included those with defined roles in virulence, stress responses, cell wall metabolism, DNA metabolism, RNA/protein synthesis and cell division. In contrast to tissue culture-based expression studies, transcripts from genes encoding enzymes involved in glycolysis were induced in vivo, while those involved in the nonoxidative phase of the pentose phosphate pathway had decreased levels of expression [44]. It is possible that these contrasting results reflect differences observed between growth conditions within tissue culture cells versus growth in whole organs and animal tissues. The observed changes in gene expression patterns clearly indicate that L. monocytogenes maintains the ability to effectively differentiate between in vitro and in vivo environmental conditions.
PrfA, the ace in the hole for L. monocytogenes intracellular survival
Abundant experimental evidence suggests that PrfA, the master virulence regulatory protein, is central to the ability of L. monocytogenes to optimize life within a mammalian host and to transition from a saprophytic life in soil [5,45]. This well-studied protein is a 27-kDa transcriptional activator that is a member of the Crp/Fnr family of transcriptional regulators [46]. PrfA activates transcription via the recognition of a 14-bp palindromic DNA binding site, also know as the PrfA box, located in the −40 region of its target promoters [47]. PrfA regulates the expression of a large number of gene products directly associated with bacterial virulence in mammals [47–49]. Strains lacking prfA are severely impaired for intracellular growth and are >100,000-fold less virulent in murine infection models, demonstrating the critical requirement of this transcriptional regulator for L. monocytogenes pathogenesis [50]. In addition to gene products required for host cell invasion, intracellular replication and cell-to-cell spread, PrfA induces the expression of a bile salt hydrolase (encoded by bsh), as well as a bile exclusion system (encoded by bilE), both of which have been shown to contribute to bacterial survival in the intestine [51–53]. Overall, PrfA is required for the expression of a number of diverse factors intimately associated with L. monocytogenes virulence and persistence.
PrfA is essential for the adaptation of L. monocytogenes to life within host cells, and the activity of this master regulator is itself carefully regulated by a variety of mechanisms (summarized in Figure 2) [47,54]. Transcriptional regulation of prfA expression occurs via three separate promoter elements. Two promoters, prfAP1 and prfAP2, are located immediately upstream of the prfA translation initiation codon, while the third promoter lies immediately upstream of plcA and results in the generation of a plcA–prfA bicistronic transcript. The prfAP1 and prfAP2 promoters direct the synthesis of monocistronic transcripts of prfA that generate the initial levels of PrfA protein required to activate expression of hly and plcA, whose gene products are needed for efficient escape of L. monocytogenes from host cell phagosomes [55]. The plcA promoter, which is activated by PrfA, directs the synthesis of the plcA–prfA transcripts, resulting in the high levels of PrfA synthesis that are required to direct actA expression for efficient bacterial cell-to-cell spread [55]. The prfAP1 promoter contains characteristics of a σA-dependent promoter, which is the primary σ-factor determining RNA polymerase specificity required for transcription in actively growing, unstressed bacterial cells [54]. The prfAP2 promoter region contains sequences that resemble a PrfA binding box, a σA-dependent promoter and the general stress response σ-factor σB-dependent promoters [55,56]. σB directs RNA polymerase to the promoter regions of a large number of genes involved in adaptation to general environmental stresses, such as conditions of low pH, high osmolarity, oxidative stress and carbon starvation [7]. A number of genes coregulated by PrfA and σB have been shown to contribute to pathogenesis of L. monocytogenes, supporting a cross-talk network between these two regulators and possibly other stress response regulators and alternative σ-factors (CtsR, HrcA, σC, σH and σL) [57]. Experimental evidence suggests that the σA- and σB-dependent prfAP1 and prfAP2 promoters are functionally redundant in vivo, as strains containing deletions of either prfAP1 or prfAP2 are fully virulent in mouse infection models; however, the presence of at least one of the promoters is required for full bacterial virulence [55].
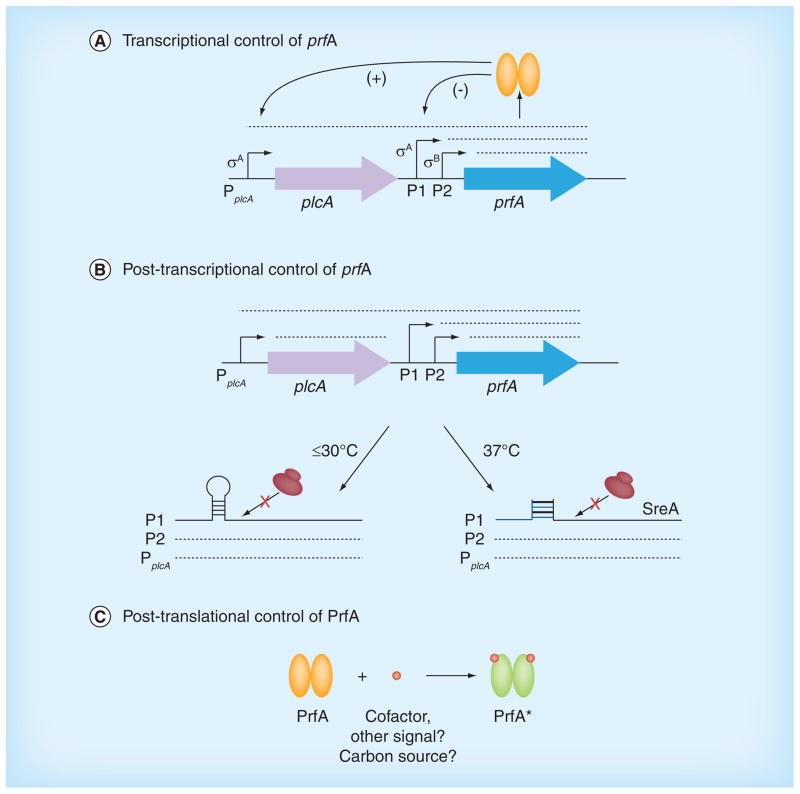
Figure 2. Multiple regulatory check-points control prfA expression and protein activity.
PrfA plays an essential role in facilitating Listeria monocytogenes survival within host cells, and the activity of this critical virulence regulator is itself tightly regulated by a number of mechanisms, including transcriptional, post-transcriptional and post-translational modes of control. (A) Transcriptional control of prfA expression is mediated by the presence of three separate promoter elements. PprfAP1 (P1) and PprfAP2 (P2) are located immediately upstream of prfA, and both direct monocistronic transcripts of prfA. The PplcA promoter is located upstream of plcA and directs both a monocistronic plcA transcript and a bicistronic plcA and prfA transcript. PprfAP1 and PprfAP2 are responsible for maintaining basal levels of PrfA protein, but both promoters are negatively (-)influenced by high levels of PrfA, whereas PplcA is positively (+) influenced, resulting in the production of the bicistronic mRNA to generate the high levels of PrfA required for intracellular growth and spread. (B) Post-transcriptional control of prfA expression involves the presence of a thermosensor riboswitch in the 5′ untranslated region of the prfAP1-directed mRNA promoter region that forms a stem-loop structure at temperatures of 30°C or lower. This stem-loop structure effectively masks the prfA mRNA ribosome-binding site to inhibit PrfA protein synthesis. At higher temperatures (37°C), the thermosensor stem-loop is destabilized; however, a trans-acting S-adenosyl methionine-responsive riboswitch (SreA) is then able to bind to a complementary region in the prfA transcript in the prfAP1 promoter region to inhibit translation and reduce PrfA protein synthesis. (C) Post-translational modification of PrfA is required to fully activate PrfA within the host. Binding of a small-molecule cofactor induces structural changes that activate PrfA and that are associated with the high levels of PrfA-dependent virulence gene expression required for survival within the host.
The second mode of prfA regulation involves post-transcriptional modification of gene expression through RNA-based mechanisms that include a riboswitch as well as an sRNA (Figure 2). Johansson et al. first identified a thermosensor riboswitch present in the 5′-UTR of the prfAP1-directed mRNA as a region that forms a stem-loop structure at temperatures of 30°C or lower to effectively mask the ribosome binding region of prfA and inhibit translation [58]. The prfAP1-directed mRNA stem-loop becomes unstable at temperatures of 37°C or higher, such that translation can occur, leading to the production of increased quantities of PrfA. The plcA and prfAP2 promoters do not appear to be subject to this mode of thermoregulation, thus transcripts from these promoters are likely to contribute to the expression of PrfA-dependent virulence genes at temperatures at or below 30°C [59].
With regard to sRNA regulation of prfA mRNA translation, Loh et al. identified a region of complementarity between a defined location of sreA, one of seven putative S-adenosyl methionine- responsive riboswitches in the L. monocytogenes transcriptome, and the distal end of the prfA 5′-UTR [60]. SreA directly interacts with the prfA 5′-UTR to reduce prfAP1-directed mRNA translation at 37°C, an observation that suggests that the prfA mRNA thermosensor represents the predominant regulation of prfAP1 transcripts at low temperatures, with SreA capable of functioning at higher temperatures that are relevant to bacterial infection of mammalian hosts (Figure 3). As an interesting side note, the first complete transcriptome analysis of L. monocytogenes revealed the existence of at least 50 encoded sRNAs, of which three were shown to be highly expressed during intracellular growth in macrophages and one that significantly contributed to virulence following oral inoculation of mice [6,61].
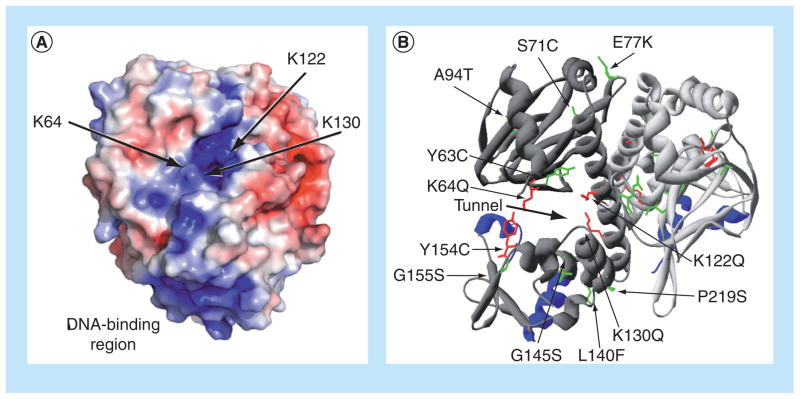
Figure 3. Location of the putative PrfA cofactor-binding pocket and of mutations that influence PrfA activation.
(A) Electrostatic modeling of wild-type PrfA protein demonstrating the potential distribution of solvent-accessible surface charges on the protein dimer and indicating binding-pocket mutations. Positive charge is shown in blue and negative charge is shown in red, with electrostatic potentials ranging from −4 kT/e (red) to +4 kT/e (blue). Arrows point to the lysine residues that contribute to the positive charge of the putative cofactor-binding pocket within PrfA. The positive charge of the DNA-binding region is also highlighted at the bottom of the PrfA monomer. (B) Ribbon modeling of PrfA, highlighting the putative cofactor-binding pocket described by Eiting et al. [62], as indicated by the thick black arrow, and identifying amino acid substitutions that influence PrfA activation. The monomers that make up the dimer are colored either light or dark gray, and the DNA-binding helix-turn-helix motifs are shown in blue. PrfA* mutations resulting in high levels of PrfA-dependent virulence gene expression are colored in green, while specific mutations abrogating or reducing PrfA activation are colored in red.
(A) Reproduced with permission from [74].
The third and possibly most important mechanism for regulating PrfA activity occurs through post-translational modification. PrfA protein belongs to the Crp–Fnr family of transcriptional regulators, of which there are approximately 400 members [46,62]. Proteins in this family usually function as dimers and generally require the binding of small-molecule cofactors (e.g., cAMP for Crp) or other forms of post-translational modification (e.g., the binding of carbon monoxide by the heme moiety of CooA) for full activity. There are several lines of evidence that suggests that PrfA is also likely to require the binding of a small-molecule cofactor for full activity. PrfA shares significant structural homology with Crp and other family members, and conditions have been described under which PrfA protein is synthesized but appears inactive (e.g., when L. monocytogenes is grown in the presence of readily metabolized carbon sources such as glucose and cellobiose) [62,63]. In addition, Ripio et al. described the identification of a L. monocytogenes strain that contained a single mutation within prfA-coding sequences that resulted in the constitutive expression of PrfA-dependent virulence genes in broth culture [64]. The substitution of a serine for a glycine at position 145 within PrfA was suggested to be analogous to an A144T mutation identified within Crp that resulted in the constitutive expression of Crp-dependent gene products (Crp* mutants). Similar to Crp*, the PrfA G145S mutation alters PrfA protein confirmation and increases the DNA binding affinity of PrfA for its target promoters via the repositioning of the helix-turn-helix DNA-binding motif [65]. PrfA G145S and other mutations that appear to constitutively activate PrfA are referred to as PrfA* mutations [64].
To date, there have been a number of additional mutations identified that result in PrfA activation. The spectrum of reported prfA* mutations include G145S, Y63C, S71C, E77K, A94T, L140F, Y154C, L148P, G155S and P219S substitution mutants (Figure 3) [66–73]. A number of these mutations map to very different regions of PrfA in comparison to the original G145S PrfA* mutation, and their influences on PrfA activity are not equivalent. Strains containing different prfA* alleles exhibit levels of actA expression in broth culture that range from fourfold to >200-fold greater than the levels of expression observed in wild-type bacteria [66,68–70]. L. monocytogenes prfA* strains also exhibit elevated levels of other PrfA-dependent gene products; however, with the exception of PrfA G145S, which has been crystallized, the mechanisms by which the other prfA* mutations confer constitutive activation are not clear. While prfA* mutations have proven useful for defining the range and extent of gene products whose expression can be influenced by PrfA, the nature of the ligand or cofactor required for PrfA activation under normal conditions remains unknown.
Eiting et al. did identify a putative cofactor-binding site in their structural model of PrfA, similar in some respects to the cofactor-binding site present in Crp [62]. This predicted PrfA cofactor-binding site was described as a tunnel-like region located between the N-terminal β-barrel and C-terminal DNA-binding domains of the protein monomer. Electrostatic modeling of this predicted binding pocket revealed a high degree of positive charge stemming from the presence of three lysine residues: K64, K122 and K130 (Figure 3a). Charge neutralization of the K64 and K122 residues via glutamine substitution impaired PrfA DNA binding and full activation of PrfA within the cytosol of infected host cells, whereas a K130 substitution completely abolished protein activity without affecting the protein levels [74]. The introduction of the prfA* G145S mutation that constitutively activates PrfA in the absence of cofactor alleviated the phenotypes conferred by the individual K64Q and K122Q substitutions, but did not restore activity for the K130Q mutant. These results suggested that the K64 and K122 mutations interfered with PrfA activation presumably by reducing cofactor binding, while mutation of K130 altered PrfA conformation such that the protein could no longer become activated [74]. While a putative PrfA-activating cofactor still remains unknown, these studies serve to implicate a role for the positive charge of the PrfA-binding pocket in the binding of a small anionic ligand.
In addition to the lysine substitution mutations within the putative PrfA cofactor-binding pocket, one additional mutation has been reported to inhibit PrfA activation within the host cytosol. The PrfA Y154 residue is located at the very end of the α-helix that contains G145, but it is oriented towards the cofactor-binding pocket. The substitution of cysteine for a tyrosine at this location (Y154C) modestly enhanced PrfA-dependent gene expression in broth culture, as well as DNA-binding activity. Despite these modest increases in PrfA activity observed in broth culture, the Y154C mutation inhibited full activation of PrfA within the cytosol and significantly attenuated bacterial virulence [71]. The Y154C mutation has thus been speculated to either interfere with cofactor binding or to stabilize the low-activity form of PrfA, thereby interfering with the conformational change necessary to confer full PrfA activation.
Forcing L. monocytogenes to show its cards: the use of prfA* to identify factors expressed within host cells
Activation of PrfA upon bacterial entry into host cells enhances intracellular bacterial fitness by increasing the expression of gene products that contribute to phagosome escape, replication and L. monocytogenes motility within the cytosol [48]. A number of the genes directly regulated by PrfA are located on a Listeria pathogenicity island referred to as LIPI-1 and include hly, plcA, prfA, mpl, actA and plcB (Figure 4), while others (inlA, inlB, inlC, bsh, prsA2 and hpt) are located elsewhere in the chromosome. The ability to mutationally activate prfA such that broth-grown cultures of L. monocytogenes can be made to express gene products normally expressed by intracellular bacteria has provided a genetic means of identifying novel virulence factors. Several studies have used microarray analyses to compare the profiles of wild-type L. monocytogenes grown in brain–heart infusion broth with those of prfA* mutants. These studies have suggested that the expression of at least 145 or more genes may be modulated by PrfA [48,75]. Milohanic et al. identified significant overlap between genes whose expression was influenced by PrfA and stress-responsive genes regulated by the stress-responsive alternative σ-factor σB [75]. However, studies by Ollinger et al. using RT-PCR reported that the transcript levels of some of PrfA-associated genes identified by Milohanic et al. [75] were not significantly affected by the presence or absence of PrfA [76]. Discrepancies between these independent studies may reflect disparities between laboratory conditions, variations between strains used for examination (EGDe versus 10403S) or additional undefined complexities associated with PrfA-dependent gene expression.
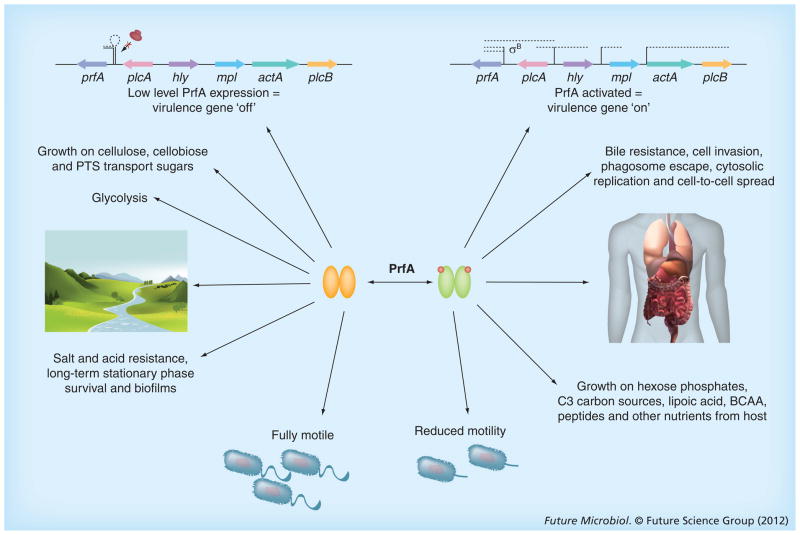
Figure 4. Listeria monocytogenes regulates PrfA activity so as to increase bacterial fitness in multiple environments.
Experimental evidence indicates that the expression and activity of PrfA must be carefully regulated in order to optimize Listeria monocytogenes fitness in diverse environments. Outside of host cells, the expression of prfA is low, as is PrfA activity, resulting in low levels of PrfA-dependent virulence gene expression. Under these conditions, the bacterium readily grows on preferred carbon sources such as glucose and cellobiose, with glycolysis being the predominant metabolic pathway. The bacteria exhibit robust flagella-mediated swimming motility, resistance to salt and acid stress and PrfA-enhanced biofilm formation on abiotic surfaces. Following entry of L. monocytogenes into a mammalian host, PrfA becomes highly activated and increases the expression and secretion of multiple gene products that enable bacterial survival within host cells. These gene products include those with direct roles in pathogenesis, as well as those that contribute to bile resistance and the metabolism of alternative carbon sources that are prevalent within the cytosol.
BCAA: Branched chain amino acid; PTS: Phosphotransferase system.
prfA* mutants have also been used as a tool to examine the effects of constitutive activation of PrfA on patterns of L. monocytogenes protein secretion. Secreted proteins are often the first bacterial factors to interact with the host, and a comparison of secreted protein profiles derived from the culture supernatants of wild-type ΔprfA and prfA* mutants identified at least 17 proteins that were differentially secreted following PrfA activation [77]. The majority of the genes encoding these proteins did not contain recognizable PrfA binding sites in their upstream promoter regions, suggesting that the synthesis and/or secretion of these proteins was indirectly influenced by PrfA activation. Proteins with increased abundance of the supernatants derived from prfA* cultures included a number of previously identified virulence factors, as well as putative ABC transporters, cell wall-modifying enzymes, antigenic lipoproteins and chaperone proteins associated with protein secretion [77]. Several of these secreted gene products that appear indirectly regulated by PrfA have been demonstrated to contribute to L. monocytogenes pathogenesis as briefly described below, and these gene products serve as further examples of the expansive influence of PrfA on L. monocytogenes life within the host [77–80].
CtaP is a multifunctional cysteine transport-associated protein whose secretion is increased following PrfA activation and contributes to bacterial adhesion to host cells, acid resistance and bacterial membrane integrity [80]. Disruption of tcsA, a secreted protein first identified as a stimulating antigen for CD4+ T cells [77], reduced bacterial virulence in mice, as did the loss of the chiA-encoded chitinase enzyme [79], which has also recently been shown to be PrfA-regulated [81]. NamA, a murein hydrolase required for bacterial cell septation during logarithmic growth, is also required for full virulence in mouse models of infection [78]. L. monocytogenes mutants lacking prsA2, encoding one of two post-translocation secretion chaperones with peptidyl–propryl isomerase activity, are severely attenuated for bacterial growth in mice and exhibit reduced viability when PrfA becomes activated, presumably due to the accumulation of misfolded proteins at the membrane–cell wall interface [82,83]. PrsA2 was also found to contribute to bacterial cell wall integrity, where it has been postulated to modify the cell wall to promote protein secretion and bacterial survival within the cytosol [84]. PrfA activation clearly influences multiple aspects of bacterial physiology by altering L. monocytogenes gene expression to optimize replication within its cytosolic niche.
Deciphering the natural in vivo cues that activate PrfA & stimulate PrfA-dependent virulence gene expression
While prfA* mutants have proven extremely useful for the identification of novel L. monocytogenes gene products that contribute to pathogenesis, the true nature of the signal(s) that triggers PrfA activation and thus adapts L. monocytogenes for intracellular life remains unknown. One promising clue is the long-noted but poorly understood linkage between PrfA-dependent virulence gene expression and available carbon sources. Bacterial growth in the presence of readily metabolized carbohydrates such as glucose or the plant-derived sugar cello biose dramatically reduces the expression of PrfA-dependent gene products [85]. These preferred carbon sources are transported into the bacterial cell via the phosphoenolpyruvate–phosphotransferase system (PTS), a multiprotein phosphorelay system that couples the transport of sugars across the bacterial membrane with simultaneous phosphorylation of the incoming sugars [86]. By contrast, growth of L. monocytogenes in the presence of carbon sources prevalent within host cells, such as glycerol or phosphorylated sugars such as glucose-1-phosphate, does not lead to the repression of PrfA-dependent virulence gene expression [36,43,87]. prfA* mutants are impaired for growth in the presence of glucose, but more readily metabolize glycogen, glycerol and other C3 compounds that serve as intracellular carbon sources [43,88]. In addition, microarray-based studies, as well as 13C-isotopologe profiling, suggest that L. monocytogenes switches its metabolic activity during growth in vivo [34,89].
How might available carbon sources modulate PrfA activity? Several reports have observed a correlation between the levels of PrfA-dependent gene expression and the phosphorylation status of selected components of the PTS permeases complex [43,85,90]. In the presence of PTS-dependent sugars, phosphorylation of incoming sugars results in the accumulation of unphosphorylated PTS sugar transport component EIIA, and the presence of the unphosphorylated EIIA correlates with a decrease in PrfA-dependent gene expression. It has thus been proposed that one or more sugar-specific, unphosphorylated EIIA component of PTS binds and sequesters PrfA, keeping PrfA functionally inactive and preventing the induction of virulence gene expression. A recent study by Ake et al. reported that mutants lacking EIIABMan (ManL), one of two man-nose transporters that functions as the major glucose transporter in L. monocytogenes, exhibit increased expression of PrfA-dependent genes [91]. Bacterial growth in the presence of non-PTS-dependent carbon sources such as hexose phosphates or glycerol results in an accumulation of phosphorylated EIIA; this form of EIIA is not thought to sequester PrfA, resulting in the full availability of PrfA to induce target gene expression [34,43,85]. Taken together, this model would suggest that PrfA differs from other Crp/Fnr family members in not requiring the binding of small-molecule signals for triggering full activity, and that distinct EIIA molecules must be capable of binding and sequestering all available PrfA within the cell [46]. However, as discussed above, significant structural and functional analysis of wild-type and PrfA* proteins suggests the presence of a small-molecule binding pocket, as well as induced conformational changes in PrfA* structure [62,63,74].
An alternative model suggests that L. monocytogenes phosphorylated PTS permeases function to stimulate the synthesis of a cofactor or secondary messenger that activates PrfA, similar to what is observed in Escherichia coli, where the glucose-specific PTS EIIA (EIIAGlc) phosphate stimulates adenylate cyclase to produce the Crp cofactor cAMP [92]. It is possible that one or more EIIA permease does indeed bind and sequester PrfA, but an additional cytosol-induced signal may then be needed for full PrfA activation following its release from EIIA. A complete picture of the interplay between carbon source utilization and PrfA activation awaits further experimental analyses. It seems tempting to speculate that L. monocytogenes deciphers its environment and the gene products it needs to survive based on what is available for the bacterium to metabolize.
Moderation is the key: why constitutive activation of PrfA is not beneficial for L. monocytogenes survival
With respect to PrfA activation within the infected host, it would appear that L. monocytogenes cannot have too much of a good thing, as prfA* strains exhibit a number of advantages over wild-type bacteria. Strains containing prfA* are hyperinvasive, mediate more efficient phagosome escape and initiate bacterial actin-based motility more rapidly [66,69,70,73,93]. Activation of PrfA also appears to shift L. monocytogenes metabolism towards the use of C3 sugars and phosphorylated sugars, the principal carbon sources used by L. monocytogenes for growth within the cytosol [89]. prfA* mutants are hyper-virulent in mouse infection models, and exhibit a competitive fitness advantage over wild-type strains during both oral and intravenous mixed infections in mice [67,70,93].
If prfA* strains reign supreme during host infection, why then is the activity of PrfA so tightly regulated, complete with multiple checkpoint mechanisms? The answer would appear to reside in the need for L. monocytogenes to carefully balance life within the host with life in the outside environment (Figure 4) [93]. Constitutively activated prfA* mutants are impaired for flagella-mediated swimming motility, a defect that would be expected to compromise bacterial fitness in environments where the bacteria must be able to detect and swim towards available nutrient sources [67,69,70,77]. The prfA*-associated swimming motility defect does not appear to be due to a defect in flagellum assembly, but rather in the ability of prfA* mutants to detect and initiate movement towards nutrient sources [70]. Flagella-mediated swimming motility has also been demonstrated to be critical for L. monocytogenes biofilm formation on abiotic surfaces [94]. Biofilm formation presumably is advantageous for the attachment and the proliferation of L. monocytogenes in many nonhost environments that might include food-processing plants, providing a potential reservoir for bacterial contamination of food products. Interestingly, although prfA* mutants exhibit modest biofilm defects, wild-type prfA contributes to biofilm formation on abiotic surfaces in a manner that it is independent of swimming motility [95]. prfA deletion mutants are fully motile but are impaired in the formation of microcolonies, an early step of biofilm development. PrfA has therefore been proposed to influence biofilm maturation after the initial attachment to a surface [95]. A requirement for PrfA for optimal bacterial biofilm formation may be one mechanism by which this regulator is maintained in the L. monocytogenes genome in environments outside of host cells.
In addition to swimming motility defects, prfA* mutants exhibit a pronounced fitness defect when grown in the presence of wild-type bacteria in mixed broth culture, despite displaying no obvious growth defects in monoculture [93]. Stress conditions such as high osmolarity or low pH exacerbate the competitive defects observed for prfA* strains in a manner that is independent of the stress-responsive σ-factor σB. prfA* strains are less proficient at using carbon sources such as glucose and cellobiose, but have an enhanced capacity for growth in the presence of C3 sugars, such a glycerol, and phosphorylated sugars, such as glucose-1-phosphate, which are the primary carbon sources supporting L. monocytogenes growth within the cytosol [43,85,93,96].
Lastly, prfA* mutations appear to negatively impact the ability of L. monocytogenes to survive long periods of starvation [97]. The phenomenon known as ‘growth advantage in stationary phase’ (GASP) has recently been described for L. monocytogenes [97]. The GASP phenotype, initially described for E. coli [98], is a process by which bacteria from an aged culture develop the ability to outcompete bacteria from a younger culture when these cultures are mixed together. GASP results from the acquisition of genetic mutations that enhance bacterial growth and survival during periods of long-term starvation. L. monocytogenes is capable of expressing a GASP phenotype that enhances long-term survival of the bacterium without negatively impacting bacterial virulence. Interestingly, L. monocytogenes prfA* mutants exhibited a diminished capacity for GASP expression for reasons that have not yet been defined [97]. It is thus readily apparent that PrfA activity represents a double-edged sword for L. monocytogenes, in that the regulator is a weapon required for successful bacterial confrontation with a eukaryotic host, but one that becomes a burden to bacterial survival away from the mammalian battlefield.
Future perspective
The L. monocytogenes fight for survival thus requires a balance between the expression of virulence factors and life within host cells with the ability of the bacterium to survive as a peaceful saprophyte in the soil. A great deal of emphasis has thus far been directed towards identifying and characterizing the L. monocytogenes gene products that contribute to life within mammalian cells; however, it is becoming increasingly important for human health and food safety to better understand how the bacterium manages to maintain its virulence arsenal while occupying habitats outside of mammalian hosts. Recent evidence, such as the indication of a role for PrfA in biofilm formation [95], suggests that at least some L. monocytogenes virulence determinants have functional roles outside of host cells. One surprising example of a multipurpose virulence factor is the secreted L. monocytogenes chitinase ChiA, which enhances bacterial growth in the presence of chitin but also contributes to virulence in mice, despite the lack of chitin synthesis in mammals [79]. The presence of other soil dwellers and potential predators, such as amoebae or nematodes, in L. monocytogenes outdoor habitats may provide additional targets for the bacterium’s virulence gene product-based defense strategies.
Be it a vegetarian saprophyte or a carnivorous intruder, L. monocytogenes is clearly an organism that has adapted itself to a wide variety of environmental conditions. Humans may feel fortunate that a relatively small number of environmental bacteria, such as L. monocytogenes, have developed the capacity to gain access to the nutrients hidden within our bodies and cells. Overall, in addition to its considerable utility as a model bacterium for understanding numerous aspects of host–pathogen interactions, cell biology and host immunity, L. monocytogenes is also an excellent model organism for studies to determine how environmental organisms develop the capacity to become pathogens. The regulatory protein PrfA is a key player in coordinating the L. monocytogenes transition between the soil and the mammalian cytosol; however, we have yet to uncover the signals that trigger the activation of PrfA upon bacterial entry into a mammalian host. Thus, while there have been many lessons learned from studies focused on L. monocytogenes physiology and pathogenesis, it seems clear that much remains to be revealed by this small but resourceful invader of human cells.
Executive summary.
The environmental bacterial pathogen Listeria monocytogenes
Listeria monocytogenes is a Gram-positive bacterium that is thought to live as a saprophyte in the soil, but which has the capacity to transition into an intracellular pathogen when ingested by mammals, where it can cause serious and sometimes fatal disease.
Life as an intracellular pathogen
L. monocytogenes life as an intracellular pathogen requires the expression of numerous gene products that promote bacterial entry in host cells, escape from the phagosome, utilization of host carbon sources and other cytosolic nutrients, actin-based motility for spread to adjacent cells and modification of the bacterial cell surface, as well as bacterial manipulation of host cytosol immune survelliance pathways.
PrfA coordinates survival of L. monocytogenes in multiple environments
PrfA is a transcriptional regulator that exists in two activity states. The highly active form induces the production of nearly all virulence factors required to promote L. monocytogenes life within the host, while the protein in its low-activity form contributes to life in outside environments. Multiple mechanisms exist to regulate prfA expression and activity, including transcriptional, post-transcriptional and post-translational methods of control.
Post-translational activation of PrfA occurs within host cells & is required for bacterial virulence
Experimental evidence strongly suggests that PrfA requires the binding of a small molecule cofactor for full activity; mutations within an identified PrfA cofactor-binding pocket impair PrfA activation in the cytosol and reduce bacterial virulence. While the identity of the PrfA cofactor remains unknown, mutations in prfA (prfA*) have been identified that lock the protein into a constitutively active form.
The appropriate regulation of PrfA activity is required for L. monocytogenes to optimize bacterial fitness in disparate environments
While constitutive activation of PrfA enhances bacterial virulence within animal models of infection, prfA* mutants are defective for activities likely to contribute to bacterial life outside of host cells, including swimming motility, resistance to stress conditions, use of exogenous carbon sources and long-term starvation survival. L. monocytogenes thus responds to physiological cues and signals to modulate PrfA activity in order to balance bacterial life both inside and outside of mammalian host cells.
Acknowledgments
The authors would like to acknowledge the excellent work done by many laboratories in the field of Listeria monocytogenes pathogenesis and we apologize to all our colleagues whose important work could not be directly cited.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Disclaimer
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding sources.
Financial & competing interests disclosure
This work was supported by Public Health Service grants AI41816 and AI083241 (NE Freitag) from NIAID. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- 1.Albert-Weissenberger C, Cazalet C, Buchrieser C. Legionella pneumophila – a human pathogen that co-evolved with fresh water protozoa. Cell Mol Life Sci. 2007;64(4):432–448. doi: 10.1007/s00018-006-6391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson EJ, Harris JB, Morris JG, Jr, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009;7(10):693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong E, Vaaje-Kolstad G, Ghosh A, et al. The Vibrio cholerae colonization factor GbpA possesses a modular structure that governs binding to different host surfaces. PLoS Pathog. 2012;8(1):e1002373. doi: 10.1371/journal.ppat.1002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czuprynski CJ. Listeria monocytogenes: silage, sandwiches and science. Anim Health Res Rev. 2005;6(2):211–217. doi: 10.1079/ahr2005111. [DOI] [PubMed] [Google Scholar]
- 5.Freitag NE, Port GC, Miner MD. Listeria monocytogenes – from saprophyte to intracellular pathogen. Nat Rev Microbiol. 2009;7(9):623–628. doi: 10.1038/nrmicro2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪▪.Toledo-Arana A, Dussurget O, Nikitas G, et al. The Listeria transcriptional landscape from saprophytism to virulence. Nature. 2009;459(7249):950–956. doi: 10.1038/nature08080. The first Listeria monocytogenes transcriptome map leading to the identification of a large number of sRNAs that may play important roles in bacterial physiology and virulence. [DOI] [PubMed] [Google Scholar]
- 7.Chaturongakul S, Raengpradub S, Wiedmann M, Boor KJ. Modulation of stress and virulence in Listeria monocytogenes. Trends Microbiol. 2008;16(8):388–396. doi: 10.1016/j.tim.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swaminathan B, Gerner-Smidt P, Whichard JM. Foodborne disease trends and reports. Foodborne Pathog Dis. 2006;3(4):316–318. doi: 10.1089/fpd.2006.3.316. [DOI] [PubMed] [Google Scholar]
- 9.Mead PS, Dunne EF, Graves L, et al. Nationwide outbreak of listeriosis due to contaminated meat. Epidemiol Infect. 2006;134(4):744–751. doi: 10.1017/S0950268805005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottlieb SL, Newbern EC, Griffin PM, et al. Multistate outbreak of listeriosis linked to turkey deli meat and subsequent changes in US regulatory policy. Clin Infect Dis. 2006;42(1):29–36. doi: 10.1086/498113. [DOI] [PubMed] [Google Scholar]
- 11.Lynch M, Painter J, Woodruff R, Braden C. Surveillance for foodborne-disease outbreaks – United States, 1998–2002. MMWR Surveill Summ. 2006;55(10):1–42. [PubMed] [Google Scholar]
- 12.Schwartz B, Ciesielski CA, Broome CV, et al. Association of sporadic listeriosis with consumption of uncooked hot dogs and undercooked chicken. Lancet. 1988;2(8614):779–782. doi: 10.1016/s0140-6736(88)92425-7. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Multistate outbreak of listeriosis associated with Jensen Farms cantaloupe – United States, August–September 2011. MMWR Morb Mortal Wkly Rep. 2011;60(39):1357–1358. [PubMed] [Google Scholar]
- 14.Stone SC, Shoenberger J. Update: multistate outbreak of listeriosis – United States, 2000. Ann Emerg Med. 2001;38(3):339–341. doi: 10.1067/mem.2001.117781. [DOI] [PubMed] [Google Scholar]
- 15▪.Camejo A, Carvalho F, Reis O, Leitao E, Sousa S, Cabanes D. The arsenal of virulence factors deployed by Listeria monocytogenes to promote its cell infection cycle. Virulence. 2011;2(5):379–394. doi: 10.4161/viru.2.5.17703. Along with [16], provide a detailed overview of L. monocytogenes gene products that contribute to bacterial life within the host. [DOI] [PubMed] [Google Scholar]
- 16▪.Dussurget O. New insights into determinants of Listeria monocytogenes virulence. Int Rev Cell Mol Biol. 2008;270:1–38. doi: 10.1016/S1937-6448(08)01401-9. Along with [15], provides a detailed overview of L. monocytogenes gene products that contribute to bacterial life within the host. [DOI] [PubMed] [Google Scholar]
- 17.Mostowy S, Cossart P. Virulence factors that modulate the cell biology of Listeria infection and the host response. Adv Immunol. 2012;113:19–32. doi: 10.1016/B978-0-12-394590-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 18▪▪.Drevets DA, Bronze MS. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol. 2008;53(2):151–165. doi: 10.1111/j.1574-695X.2008.00404.x. Well-written review of L. monocytogenes invasive disease. [DOI] [PubMed] [Google Scholar]
- 19.Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes Infect. 2007;9(10):1236–1243. doi: 10.1016/j.micinf.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Bortolussi R. Listeriosis: a primer. CMAJ. 2008;179(8):795–797. doi: 10.1503/cmaj.081377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecuit M. Human listeriosis and animal models. Microbes Infect. 2007;9(10):1216–1225. doi: 10.1016/j.micinf.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Serbina NV, Shi C, Pamer EG. Monocyte-mediated immune defense against murine Listeria monocytogenes infection. Adv Immunol. 2012;113:119–134. doi: 10.1016/B978-0-12-394590-7.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alonzo F, 3rd, Bobo LD, Skiest DJ, Freitag NE. Evidence for subpopulations of Listeria monocytogenes with enhanced invasion of cardiac cells. J Med Microbiol. 2011;60(Pt 4):423–434. doi: 10.1099/jmm.0.027185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antolin J, Gutierrez A, Segoviano R, Lopez R, Ciguenza R. Endocarditis due to Listeria: description of two cases and review of the literature. Eur J Intern Med. 2008;19(4):295–296. doi: 10.1016/j.ejim.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Hardy J, Chu P, Contag CH. Foci of Listeria monocytogenes persist in the bone marrow. Dis Model Mech. 2009;2(1–2):39–46. doi: 10.1242/dmm.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy J, Francis KP, Deboer M, Chu P, Gibbs K, Contag CH. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science. 2004;303(5659):851–853. doi: 10.1126/science.1092712. [DOI] [PubMed] [Google Scholar]
- 27.Gaillard JL, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from Gram-positive cocci. Cell. 1991;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 28.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16(2):251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 29.Wadsworth SJ, Goldfine H. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect Immun. 1999;67(4):1770–1778. doi: 10.1128/iai.67.4.1770-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vazquez-Boland JA, Kocks C, Dramsi S, et al. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60(1):219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquis H, Doshi V, Portnoy DA. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63(11):4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 2007;9(10):1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs TM, Eisenreich W, Kern T, Dandekar T. Toward a systemic understanding of Listeria monocytogenes metabolism during infection. Front Microbiol. 2012;3:23. doi: 10.3389/fmicb.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph B, Przybilla K, Stuhler C, et al. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J Bacteriol. 2006;188(2):556–568. doi: 10.1128/JB.188.2.556-568.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatterjee SS, Hossain H, Otten S, et al. Intracellular gene expression profile of Listeria monocytogenes. Infect Immun. 2006;74(2):1323–1338. doi: 10.1128/IAI.74.2.1323-1338.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chico-Calero I, Suarez M, Gonzalez-Zorn B, et al. Hpt, a bacterial homolog of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc Natl Acad Sci USA. 2002;99(1):431–436. doi: 10.1073/pnas.012363899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keeney K, Colosi L, Weber W, O’Riordan M. Generation of branched-chain fatty acids through lipoate-dependent metabolism facilitates intracellular growth of Listeria monocytogenes. J Bacteriol. 2009;191(7):2187–2196. doi: 10.1128/JB.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keeney KM, Stuckey JA, O’Riordan MX. LplA1-dependent utilization of host lipoyl peptides enables Listeria cytosolic growth and virulence. Mol Microbiol. 2007;66(3):758–770. doi: 10.1111/j.1365-2958.2007.05956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marquis H, Bouwer HG, Hinrichs DJ, Portnoy DA. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect Immun. 1993;61(9):3756–3760. doi: 10.1128/iai.61.9.3756-3760.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Rajabian T, Gavicherla B, Heisig M, et al. The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-to-cell spread of. Listeria Nat Cell Biol. 2009;11(10):1212–1218. doi: 10.1038/ncb1964. Intrguing example of the ability of L. monocytogenes to spread in order to infect adjacent cells through the relief of cortical tension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cossart P. Illuminating the landscape of host–pathogen interactions with the bacterium Listeria monocytogenes. Proc Natl Acad Sci USA. 2011;108(49):19484–19491. doi: 10.1073/pnas.1112371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joseph B, Mertins S, Stoll R, et al. Glycerol metabolism and PrfA activity in Listeria monocytogenes. J Bacteriol. 2008;190(15):5412–5430. doi: 10.1128/JB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camejo A, Buchrieser C, Couve E, et al. In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathog. 2009;5(5):e1000449. doi: 10.1371/journal.ppat.1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.De Las Heras A, Cain RJ, Bielecka MK, Vazquez-Boland JA. Regulation of Listeria virulence: PrfA master and commander. Curr Opin Microbiol. 2011;14(2):118–127. doi: 10.1016/j.mib.2011.01.005. Well-written review of the contributions of PrfA to L. monocytogenes pathogenesis. [DOI] [PubMed] [Google Scholar]
- 46.Korner H, Sofia HJ, Zumft WG. Phylogeny of the bacterial superfamily of Crp–Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol Rev. 2003;27(5):559–592. doi: 10.1016/S0168-6445(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 47.Freitag NE. From hot dogs to host cells: how the bacterial pathogen Listeria monocytogenes regulates virulence gene expression. Future Microbiol. 2006;1:89–101. doi: 10.2217/17460913.1.1.89. [DOI] [PubMed] [Google Scholar]
- 48.Scortti M, Monzo HJ, Lacharme-Lora L, Lewis DA, Vazquez-Boland JA. The PrfA virulence regulon. Microbes Infect. 2007;9(10):1196–1207. doi: 10.1016/j.micinf.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Vazquez-Boland JA, Kuhn M, Berche P, et al. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14(3):584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mengaud J, Dramsi S, Gouin E, Vazquez-Boland JA, Milon G, Cossart P. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol Microbiol. 1991;5(9):2273–2283. doi: 10.1111/j.1365-2958.1991.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 51.Begley M, Sleator RD, Gahan CG, Hill C. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect Immun. 2005;73(2):894–904. doi: 10.1128/IAI.73.2.894-904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dussurget O, Cabanes D, Dehoux P, et al. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol Microbiol. 2002;45(4):1095–1106. doi: 10.1046/j.1365-2958.2002.03080.x. [DOI] [PubMed] [Google Scholar]
- 53.Sleator RD, Wemekamp-Kamphuis HH, Gahan CG, Abee T, Hill C. A PrfA-regulated bile exclusion system (BilE) is a novel virulence factor in Listeria monocytogenes. Mol Microbiol. 2005;55(4):1183–1195. doi: 10.1111/j.1365-2958.2004.04454.x. [DOI] [PubMed] [Google Scholar]
- 54.Gray MJ, Freitag NE, Boor KJ. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr Jekyll to pathogenic Mr Hyde. Infect Immun. 2006;74(5):2505–2512. doi: 10.1128/IAI.74.5.2505-2512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freitag NE, Portnoy DA. Dual promoters of the Listeria monocytogenes prfA transcriptional activator appear essential in vitro but are redundant in vivo. Mol Microbiol. 1994;12(5):845–853. doi: 10.1111/j.1365-2958.1994.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 56.Rauch M, Luo Q, Muller-Altrock S, Goebel W. SigB-dependent in vitro transcription of prfA and some newly identified genes of Listeria monocytogenes whose expression is affected by PrfA in vivo. J Bacteriol. 2005;187(2):800–804. doi: 10.1128/JB.187.2.800-804.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaturongakul S, Raengpradub S, Palmer ME, et al. Transcriptomic and phenotypic analyses identify coregulated, overlapping regulons among PrfA, CtsR, HrcA, and the alternative sigma factors sigmaB, sigmaC, sigmaH, and sigmaL in Listeria monocytogenes. Appl Environ Microbiol. 2011;77(1):187–200. doi: 10.1128/AEM.00952-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansson J, Mandin P, Renzoni A, Chiaruttini C, Springer M, Cossart P. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell. 2002;110(5):551–561. doi: 10.1016/s0092-8674(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 59.Mansfield BE, Dionne MS, Schneider DS, Freitag NE. Exploration of host–pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell Microbiol. 2003;5(12):901–911. doi: 10.1046/j.1462-5822.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 60.Loh E, Dussurget O, Gripenland J, et al. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell. 2009;139(4):770–779. doi: 10.1016/j.cell.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 61.Mraheil MA, Billion A, Mohamed W, et al. The intracellular sRNA transcriptome of Listeria monocytogenes during growth in macrophages. Nucleic Acids Res. 2011;39(10):4235–4248. doi: 10.1093/nar/gkr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62▪▪.Eiting M, Hageluken G, Schubert WD, Heinz DW. The mutation G145S in PrfA, a key virulence regulator of Listeria monocytogenes, increases DNA-binding affinity by stabilizing the HTH motif. Mol Microbiol. 2005;56(2):433–446. doi: 10.1111/j.1365-2958.2005.04561.x. First crystal structure of PrfA and PrfA* and identification of the conformational changes imposed on PrfA by the G145S activating mutation. [DOI] [PubMed] [Google Scholar]
- 63.Vega Y, Dickneite C, Ripio MT, et al. Functional similarities between the Listeria monocytogenes virulence regulator PrfA and cyclic AMP receptor protein: the PrfA* (Gly145Ser) mutation increases binding affinity for target DNA. J Bacteriol. 1998;180(24):6655–6660. doi: 10.1128/jb.180.24.6655-6660.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ripio MT, Dominguez-Bernal G, Lara M, Suarez M, Vazquez-Boland JA. A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J Bacteriol. 1997;179(5):1533–1540. doi: 10.1128/jb.179.5.1533-1540.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Youn H, Kerby RL, Conrad M, Roberts GP. Study of highly constitutively active mutants suggests how cAMP activates cAMP receptor protein. J Biol Chem. 2006;281(2):1119–1127. doi: 10.1074/jbc.M509421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miner MD, Port GC, Bouwer HG, Chang JC, Freitag NE. A novel prfA mutation that promotes Listeria monocytogenes cytosol entry but reduces bacterial spread and cytotoxicity. Microb Pathog. 2008;45(4):273–281. doi: 10.1016/j.micpath.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shetron-Rama LM, Mueller K, Bravo JM, Bouwer HG, Way SS, Freitag NE. Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol Microbiol. 2003;48(6):1537–1551. doi: 10.1046/j.1365-2958.2003.03534.x. [DOI] [PubMed] [Google Scholar]
- 68.Vega Y, Rauch M, Banfield MJ, et al. New Listeria monocytogenes prfA* mutants, transcriptional properties of PrfA* proteins and structure–function of the virulence regulator PrfA. Mol Microbiol. 2004;52(6):1553–1565. doi: 10.1111/j.1365-2958.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 69.Wong KK, Freitag NE. A novel mutation within the central Listeria monocytogenes regulator PrfA that results in constitutive expression of virulence gene products. J Bacteriol. 2004;186(18):6265–6276. doi: 10.1128/JB.186.18.6265-6276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xayarath B, Smart JI, Mueller KJ, Freitag NE. A novel C-terminal mutation resulting in constitutive activation of the Listeria monocytogenes central virulence regulatory factor PrfA. Microbiology. 2011;157(Pt 11):3138–3149. doi: 10.1099/mic.0.049957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miner MD, Port GC, Freitag NE. Functional impact of mutational activation on the Listeria monocytogenes central virulence regulator PrfA. Microbiology. 2008;154(Pt 11):3579–3589. doi: 10.1099/mic.0.2008/021063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monk IR, Gahan CG, Hill C. Tools for functional postgenomic analysis of Listeria monocytogenes. Appl Environ Microbiol. 2008;74(13):3921–3934. doi: 10.1128/AEM.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mueller KJ, Freitag NE. Pleiotropic enhancement of bacterial pathogenesis resulting from the constitutive activation of the Listeria monocytogenes regulatory factor PrfA. Infect Immun. 2005;73(4):1917–1926. doi: 10.1128/IAI.73.4.1917-1926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪.Xayarath B, Volz KW, Smart JI, Freitag NE. Probing the role of protein surface charge in the activation of PrfA, the central regulator of Listeria monocytogenes pathogenesis. PLoS One. 2011;6(8):e23502. doi: 10.1371/journal.pone.0023502. Mutational analysis of the PrfA cofactor-binding pocket, supporting a role for this region in PrfA activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milohanic E, Glaser P, Coppee JY, et al. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol Microbiol. 2003;47(6):1613–1625. doi: 10.1046/j.1365-2958.2003.03413.x. [DOI] [PubMed] [Google Scholar]
- 76.Ollinger J, Wiedmann M, Boor KJ. SigmaB- and PrfA-dependent transcription of genes previously classified as putative constituents of the Listeria monocytogenes PrfA regulon. Foodborne Pathog Dis. 2008;5(3):281–293. doi: 10.1089/fpd.2008.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Port GC, Freitag NE. Identification of novel Listeria monocytogenes secreted virulence factors following mutational activation of the central virulence regulator, PrfA. Infect Immun. 2007;75(12):5886–5897. doi: 10.1128/IAI.00845-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alonzo F, 3rd, Mcmullen PD, Freitag NE. Actin polymerization drives septation of Listeria monocytogenes namA hydrolase mutants, demonstrating host correction of a bacterial defect. Infect Immun. 2011;79(4):1458–1470. doi: 10.1128/IAI.01140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chaudhuri S, Bruno JC, Alonzo F, 3rd, Xayarath B, Cianciotto NP, Freitag NE. Contribution of chitinases to Listeria monocytogenes pathogenesis. Appl Environ Microbiol. 2010;76(21):7302–7305. doi: 10.1128/AEM.01338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xayarath B, Marquis H, Port GC, Freitag NE. Listeria monocytogenes CtaP is a multifunctional cysteine transport-associated protein required for bacterial pathogenesis. Mol Microbiol. 2009;74(4):956–973. doi: 10.1111/j.1365-2958.2009.06910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Larsen MH, Leisner JJ, Ingmer H. The chitinolytic activity of Listeria monocytogenes EGD is regulated by carbohydrates but also by the virulence regulator PrfA. Appl Environ Microbiol. 2010;76(19):6470–6476. doi: 10.1128/AEM.00297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alonzo F, 3rd, Freitag NE. Listeria monocytogenes PrsA2 is required for virulence factor secretion and bacterial viability within the host cell cytosol. Infect Immun. 2010;78(11):4944–4957. doi: 10.1128/IAI.00532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Forster BM, Marquis H. Protein transport across the cell wall of monoderm Gram-positive bacteria. Mol Microbiol. 2012;84(3):405–413. doi: 10.1111/j.1365-2958.2012.08040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alonzo F, 3rd, Xayarath B, Whisstock JC, Freitag NE. Functional analysis of the Listeria monocytogenes secretion chaperone PrsA2 and its multiple contributions to bacterial virulence. Mol Microbiol. 2011;80(6):1530–1548. doi: 10.1111/j.1365-2958.2011.07665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stoll R, Mertins S, Joseph B, Muller-Altrock S, Goebel W. Modulation of PrfA activity in Listeria monocytogenes upon growth in different culture media. Microbiology. 2008;154(Pt 12):3856–3876. doi: 10.1099/mic.0.2008/018283-0. [DOI] [PubMed] [Google Scholar]
- 86.Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70(4):939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ripio MT, Brehm K, Lara M, Suarez M, Vazquez-Boland JA. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J Bacteriol. 1997;179(22):7174–7180. doi: 10.1128/jb.179.22.7174-7180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eisenreich W, Dandekar T, Heesemann J, Goebel W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Microbiol. 2010;8(6):401–412. doi: 10.1038/nrmicro2351. [DOI] [PubMed] [Google Scholar]
- 89.Eylert E, Schar J, Mertins S, et al. Carbon metabolism of Listeria monocytogenes growing inside macrophages. Mol Microbiol. 2008;69(4):1008–1017. doi: 10.1111/j.1365-2958.2008.06337.x. [DOI] [PubMed] [Google Scholar]
- 90.Mertins S, Joseph B, Goetz M, et al. Interference of components of the phosphoenolpyruvate phosphotransferase system with the central virulence gene regulator PrfA of Listeria monocytogenes. J Bacteriol. 2007;189(2):473–490. doi: 10.1128/JB.00972-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ake FM, Joyet P, Deutscher J, Milohanic E. Mutational analysis of glucose transport regulation and glucose-mediated virulence gene repression in Listeria monocytogenes. Mol Microbiol. 2011;81(1):274–293. doi: 10.1111/j.1365-2958.2011.07692.x. [DOI] [PubMed] [Google Scholar]
- 92.Gorke B, Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6(8):613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 93▪.Bruno JC, Jr, Freitag NE. Constitutive activation of PrfA tilts the balance of Listeria monocytogenes fitness towards life within the host versus environmental survival. PLoS One. 2010;5(12):e15138. doi: 10.1371/journal.pone.0015138. Investigates the advantages and disadvantages of PrfA activation with respect to L. monocytogenes fitness inside and outside of host cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lemon KP, Higgins DE, Kolter R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J Bacteriol. 2007;189(12):4418–4424. doi: 10.1128/JB.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lemon KP, Freitag NE, Kolter R. The virulence regulator PrfA promotes biofilm formation by Listeria monocytogenes. J Bacteriol. 2010;192(15):3969–3976. doi: 10.1128/JB.00179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joseph B, Goebel W. Life of Listeria monocytogenes in the host cells’ cytosol. Microbes Infect. 2007;9(10):1188–1195. doi: 10.1016/j.micinf.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 97.Bruno JC, Jr, Freitag NE. Listeria monocytogenes adapts to long-term stationary phase survival without compromising bacterial virulence. FEMS Microbiol Lett. 2011;323(2):171–179. doi: 10.1111/j.1574-6968.2011.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Finkel SE. Long-term survival during stationary phase: evolution and the GASP phenotype. Nat Rev Microbiol. 2006;4(2):113–120. doi: 10.1038/nrmicro1340. [DOI] [PubMed] [Google Scholar]