Abstract
β-catenin, a key transducer molecule of Wnt signaling, is required for adult hair follicle growth and regeneration. However, the cellular source of Wnt ligands required for Wnt/β-catenin activation during anagen induction is unknown. In this study, we genetically deleted Wntless, a gene required for Wnt ligand secretion by Wnt-producing cells, specifically in the hair follicle epithelium during telogen phase. We show that epithelial Wnt ligands are required for anagen, as loss of Wntless in the follicular epithelium resulted in a profound hair cycle arrest. Both the follicular epithelium and dermal papilla showed markedly decreased Wnt/β-catenin signaling during anagen induction compared to control hair follicles. Surprisingly, hair follicle stem cells that are responsible for hair regeneration maintained expression of stem cell markers but exhibited significantly reduced proliferation. Finally, we demonstrate that epidermal Wnt ligands are critical for adult wound-induced de novo hair formation. Collectively, these data show that Wnt ligands secreted by the hair follicle epithelium are required for adult hair follicle regeneration and provide new insight into potential cellular targets for the treatment of hair disorders such as alopecia.
Introduction
Hair follicle growth in adult mammals occurs through cycles of growth (anagen), regression (catagen), and rest (telogen). The ability to continuously cycle through these phases is dependent on hair follicle stem cells (HFSCs), which reside in a specialized niche of the telogen hair follicle that consists of the bulge and secondary hair germ (sHG) (Cotsarelis et al., 1990; Greco et al., 2009; Myung and Ito, 2012). Remarkably, hair follicles can also regenerate de novo in adult mice following wound repair in a manner that recapitulates embryonic hair follicle development (Ito et al., 2007). Similar to embryonic hair development, signals that coordinate the growth and activation of follicular epithelial cells during both anagen onset and adult hair neogenesis involve interactions between follicular epithelial cells as well as their heterotypic interactions with adjacent mesenchymal cells in the dermis (Millar, 2002; Schmidt-Ullrich and Paus, 2005). Characterization of the molecular signals that govern adult hair follicle growth and regeneration has been the focus of recent efforts to understand both the regulation of hair growth by somatic stem cells and how intercellular interactions are coordinated to promote adult organ regeneration.
Wnt/β-catenin signaling is a central signaling pathway that regulates embryonic and adult hair follicle growth and is mediated by the intracellular molecule β-catenin, which functions in both the regulation of cell-cell adhesion and Wnt-dependent signal transduction (Huelsken et al., 2001; Lowry et al., 2005; Zhang et al., 2009). Secreted extracellular Wnt ligands activate Wnt signaling, leading to stabilization of β-catenin and its translocation to the nucleus where it binds TCF/LEF transcription factors. This complex regulates transcription of downstream target genes responsible for the pleiomorphic functions of Wnt signaling in proliferation, differentiation and migration (van Amerongen and Nusse, 2009). Previous studies showed that Wnt/β-catenin signaling is active in the hair follicle during both embryonic hair morphogenesis and postnatal anagen phase. During initiation of hair follicle development, Wnt/β-catenin signaling is first upregulated uniformly in the upper dermis and then focally in both the epithelial hair follicle placode and underlying dermal condensate (Chen et al., 2012; DasGupta and Fuchs, 1999; Zhang et al., 2009). During the adult hair cycle, Wnt activity is observed in the sHG during anagen onset and in the precortex of anagen follicles during hair shaft differentiation (DasGupta and Fuchs, 1999; Greco et al., 2009; Rabbani et al., 2011). How this dynamic pattern of Wnt signaling is regulated during hair follicle development and the adult hair cycle is still unclear.
Several studies have demonstrated a required role for Wnt/β-catenin activation in hair follicle growth. Functional studies have shown that activation of Wnt/β-catenin in the epidermis is critical for initiation of embryonic hair follicle development and for upregulation of Wnt signaling in the dermal condensate (Andl et al., 2002; Huelsken et al., 2001; Nguyen et al., 2009; Zhang et al., 2009). Similarly, wound-induced hair neogenesis (WIHN) is also dependent upon Wnt/β-catenin signaling, lending support to the theory that embryonic programs of morphogenesis are reactivated to promote regeneration in adult mammals (Ito et al., 2007).
In the adult hair cycle, progressive deletion of β catenin in the epidermis resulted in loss of contact between the follicular epithelium and dermal papilla (DP) with formation of epithelial cysts that expressed markers of interfollicular epidermal differentiation (Huelsken et al., 2001). In another study, deletion of epidermal β catenin specifically at first telogen phase resulted in hyperproliferation of HFSCs concomitant with their depletion from the niche and failure to enter anagen, suggesting that Wnt/β-catenin signaling is necessary for HFSC quiescence and maintenance (Lowry et al., 2005). Conversely, forced expression of a constitutively active β-catenin in the epidermis is sufficient to induce anagen onset and de novo hair follicle formation in adult mice (Gat et al., 1998; Lo Celso et al., 2004; Lowry et al., 2005; Van Mater et al., 2003). These studies established a critical role for epithelial β-catenin in hair growth. More recently, a cell-autonomous requirement for β-catenin in DP cells to support the growth of follicular epithelial cells during anagen was also demonstrated, revealing an essential role for Wnt activation in both the hair follicle epithelium and mesenchyme during the hair cycle (Enshell-Seijffers D, 2010).
Despite evidence demonstrating the importance of Wnt/β-catenin signaling in adult hair follicle biology, some questions remain unresolved. First, the requirement for Wnt signaling during anagen onset has not been tested independently of β-catenin’s function in adherens junctions or independently of TCF/LEF transcription factors that have Wnt-dependent and Wnt-independent functions (Huelsken et al., 2001; Lowry et al., 2005; Merrill et al., 2001; Nguyen et al., 2009; Niemann et al., 2002). In particular, a Wnt signaling mediator upstream of β-catenin has not been formally examined during anagen induction in the adult hair cycle. Additionally, it is unclear how Wnt signaling is initiated during anagen onset. Several Wnt ligands are upregulated in the sHG and less prominently in the DP during anagen (Greco et al., 2009; Rabbani et al., 2011; Reddy et al., 2001). Partly due to functional redundancy between these Wnt ligands, no study has dissected the specific requirement for Wnt ligands secreted by the hair follicle epithelium to activate Wnt signaling in both the follicular epithelium and DP during anagen onset.
To address these questions, we deleted Wntless (Wls), a gene required for the secretion of Wnt ligands by Wnt-producing cells (Banziger et al., 2006; Bartscherer et al., 2006; Goodman et al., 2006). In contrast to overexpression of DKK1, a diffusible protein that inhibits Wnt/β-catenin activation transdominantly, deletion of Wls provides a tool to inhibit Wnt-responsive cells that are physiologically activated by epithelial Wnt ligands. We show that loss of Wls specifically in HFSCs results in inhibition of Wnt/β-catenin activity in the sHG and DP with concomitant hair cycle arrest at telogen/early anagen during both spontaneous and depilation-induced anagen.
In contrast to results obtained from previous studies in which β-catenin is depleted (Huelsken et al., 2001; Lowry et al., 2005; Nguyen et al., 2009), loss of follicular epithelial Wnt ligands leads to markedly decreased HFSC proliferation, while expression of HFSC markers are maintained. Additionally, WIHN fails to occur in the absence of epidermal Wnt ligands. Altogether, these data suggest that Wnt ligands secreted by the hair follicle epithelium are essential to drive early events in adult hair follicle growth and regeneration.
Results
Wntless is upregulated in the hair follicle epithelium and dermal papilla during anagen
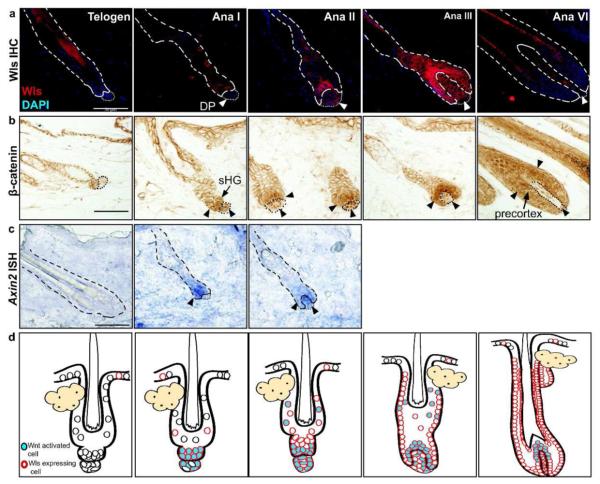
To begin to address the requirement for epidermal Wls in promoting anagen onset, we first characterized the expression of Wls in the hair follicle during telogen and anagen phases. During telogen, the hair follicle epithelium and dermal papilla (DP) express Wls at very low or undetectable levels (Figure 1a). During early anagen phases (anagen I/II) induced by depilation, Wls expression became markedly upregulated initially in the sHG and then in both the sHG and DP. By anagen III, Wls was highly expressed in the DP, epithelial cells surrounding the DP, and outer root sheath (ORS) cells. By anagen VI, elevated levels of Wls were maintained in the ORS, differentiating cells of the inner root sheath and precortex, and the DP.
Figure 1.
Wntless expression correlates with Wnt activation during anagen. (a) Wildtype (P60) club hairs were left either unplucked (telogen) or plucked to induce anagen and harvested at either 2 days (Ana I), 3 days (Ana II), 4 days (Ana III), or 9 days (Ana VI) post-depilation. Sections were analyzed for Wls expression by immunofluorescence. (b) Brightfield immunohistochemical detection of β-catenin during telogen and anagen phases as described above. Cells positive for nuclear β-catenin (arrowheads) in epithelium and DP. (c) In situ hybridization detection of Axin2 mRNA transcripts was used to detect Wnt activation on cryosections of telogen and depilation-induced early anagen phase hair follicles. (d) Illustrations of the temporospatial distribution of Wls expression and Wnt activation during telogen and anagen phases. Bar=50Om.
We sought to correlate the location of Wls expression with Wnt activity during the resting and growth phases of the hair cycle. A recent study showed that nuclear β-catenin is first detected in the sHG at the telogen-anagen transition, suggesting that Wnt activation initially occurs in the epithelium early during anagen onset (Greco et al., 2009). We further characterized changes in nuclear β-catenin immunoreactivity throughout anagen. During depilation-induced anagen, nuclear β-catenin was detected in both the sHG and DP during early anagen (Figure 1b). Consistent with previous studies, by anagen III, Wnt activated cells became localized to cells adjacent to the DP, corresponding to the forming matrix/precortex (DasGupta and Fuchs, 1999). At this stage, nuclear β-catenin became downregulated in the DP compared to early anagen DP cells. By anagen VI, nuclear β-catenin immunoreactivity was most prominent in the precortex and differentiating cells of the hair shaft, while the DP continued to show lower and more heterogeneous levels of nuclear β-catenin. In situ hybridization detection of Axin2 mRNA, a direct downstream transcriptional target and marker of Wnt signaling (Jho et al., 2002), showed that Axin2 expression was first evident in the sHG during early anagen and in both the DP and sHG by anagen II (Figure 1c). Similar expression patterns of Wls and nuclear β-catenin were also seen during spontaneous anagen (Figure S1). These results suggest that Wnt ligands are secreted by the follicular epithelium during anagen onset and then by both epithelial and mesenchymal components of the hair follicle during later stages of anagen. This expression pattern overlaps with the timing and location of Wnt activity in the hair follicle (Figure 1d).
Epidermal Wntless is required for anagen phase
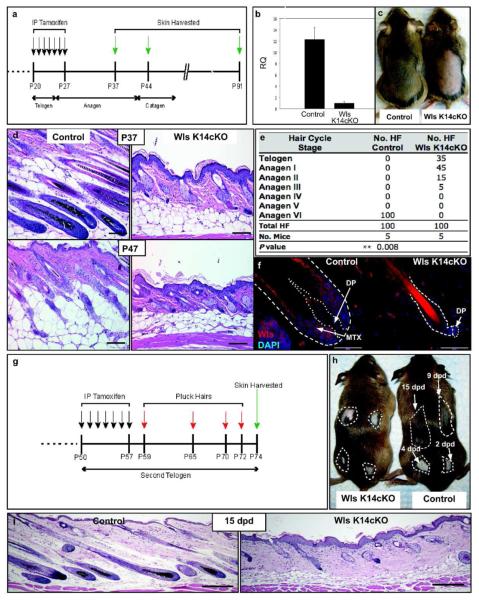
To determine if epidermal Wnt ligands are required for the hair cycle growth phase, we deleted Wls expression specifically in the basal layer of the epidermis and hair follicle, using K14-CreER;Wlsfl/fl (Wls K14cKO) mice (Carpenter et al., 2010; Vasioukhin et al., 1999). Cre-mediated recombination of the Wlsfl/fl allele was induced during the first telogen phase (Figure 2a). Quantitative PCR (qPCR) analysis of epidermal preparations showed significantly decreased Wls mRNA in induced Wls K14cKO skin compared to control skin (Figure 2b).
Figure 2.
Epidermal Wls is required for anagen. (a) Tamoxifen-mediated Cre induction regimen. (b) Relative quantities of Wls mRNA determined by qPCR from RNA isolated from dorsal skin epidermis of control and Wls K14cKO mice 5 days after induction (P32, N=5 mice). (c) Images of P37 mice shaved after induction. (d) H&E sections from control mice during anagen (P37) and catagen (P47; bar=100 μm). Wls K14cKO hair follicles at the same time points remained arrested in telogen or anagen I/II. (e) Hair cycle distribution of control and mutant mice at P37-40. (f) Wls expression in P37 control and mutant hair follicles (bar=50 μm). Scattered Wls immunoreactive cells were noted throughout the dermis but similar between control and mutant mice. (g,h) Tamoxifen was administered during second telogen prior to depilation at indicated times. (i) H&E sections from skin plucked 15 days post-depilation (15 dpd; bar=200 μm).
To determine if deletion of epidermal Wls expression affects anagen onset, skin from Wls K14cKO and control mice was examined 10-14 days after induction. At P37, control mice showed darker skin from new hair growth while skin of Wls K14cKO mice remained pink, reflecting lack of hair growth (Figure 2c). Histologically, control littermate hair follicles had entered anagen VI by P37, whereas most Wls K14cKO hair follicles were conspicuously arrested at telogen or early anagen phases (Figure 2d). This arrest was still apparent by P47 when control hair follicles had entered catagen. Overall, 80% of hair follicles from P35-37 Wls K14cKO mice were arrested in telogen and anagen I phases, while 100% of hair follicles from littermate controls progressed to anagen VI (Figure 2e). Consistent with qPCR results, mutant hair follicles showed markedly lower Wls expression immunohistochemically compared to controls (Figure 2f).
Histologically, mutant hair follicles showed a club hair surrounded by a two-layer epithelial sac corresponding to the bulge. Those in telogen exhibited a compact cluster of cells forming the sHG, which rested adjacent to the DP. Those that had progressed to early anagen showed elongation and widening of the sHG, with those in anagen II beginning to encase the DP. There was apparent hyperplasia of both the epidermis and sebaceous glands in the mutant mice, although the interfollicular epidermis of mutant mice showed normal differentiation by H&E and marker analyses (Figure 2d,i, 3e,f). When mice were analyzed at a later time point (P91), Wls K14cKO hair follicles remained largely arrested at either first telogen or anagen I phases, while control hair follicles were in the second telogen phase (Figure S2a).
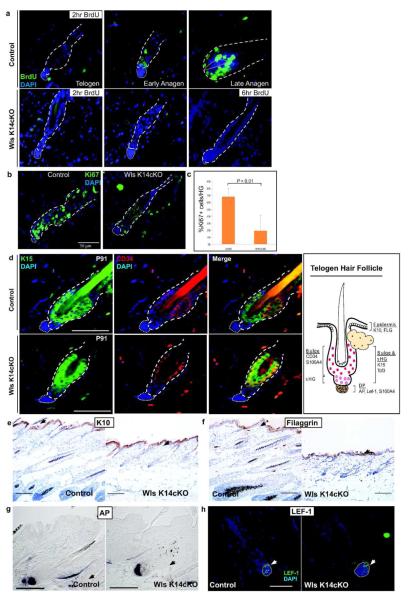
Figure 3.
Epidermal Wnts are required for HFSC proliferation but not HFSC maintenance. (a) P37 Wls K14cKO and depilated control skin were harvested 2 hr or 6 hr after BrdU administration. Virtually no BrdU+ cells were detected immunohistochemically in the bulge/sHG of Wls K14 cKO mice even after 6hr pulse. (b,c) Ki67 immunohistochemistry of depilation-induced stage-matched control follicles compared to Wls K14cKO follicles. Graph represents results from 3 mice per group. (d) Double immunofluorescent detection of HFSC markers, CD34 and K15, in control (top panels) and Wls K14cKO (bottom) mice at P91. Illustration (right) of marker expression in normal telogen skin. (e) Immunohistochemistry of interfollicular epidermal differentiation markers K10 and (f) filaggrin (black arrows; bar=100Om). (g) AP and (h) LEF-1 expression are maintained in the DP of mutant hair follicles (arrows). Black bars=100μm, white bars=50μm.
As the exact timing of anagen onset during the spontaneous hair cycle varies between individual mice, we determined if the above hair cycle defects could be observed following experimentally-induced anagen. Depilation of skin during second telogen phase is a potent stimulator of anagen onset (Ito et al., 2002). Wls K14cKO and control mice were induced after entering second telogen (P50) and then depilated (Figure 2g). Histological sections from depilated areas of control mice showed a gradual progression of anagen phases in which the earliest depilated area exhibited plucked hair follicles in anagen VI. By contrast, most plucked hair follicles of Wls K14cKO mice remained in either telogen or anagen I/II phases within all depilated areas, and only few hair follicles advanced to later stages of anagen (Figure 2h,i, S2b). Collectively, these data suggest that epithelial Wnt ligands are essential for initiation of anagen during the hair cycle.
Wntless-deficient hair follicles show decreased proliferation
During the telogen-to-anagen transition, sHG cells proliferate to initiate the growth phase of the hair follicle (Greco et al., 2009). We analyzed proliferation in Wls K14cKO and control hair follicles. Bromodeoxyuridine (BrdU) nucleotide uptake by mutant hair follicles was compared to control hair follicles that were either in telogen or depilated to induce early or late anagen (Figure 3a). As expected, control telogen hair follicles showed no BrdU uptake after a 2-hour pulse. By early anagen, many cells within the sHG were BrdU+, and by late anagen, most cells within the matrix were BrdU+. In contrast, none of the arrested hair follicles in Wls K14cKO mice showed BrdU+ cells. Even after a longer pulse of 6 hours, most Wls K14cKO hair follicles showed no BrdU+ cells (Figure 3a), and only rare hair follicles that appeared to be in anagen I showed one BrdU+ cell within the sHG (data not shown). When Ki67 immunohistochemistry was used to assess proliferation, Wls K14cKO mice showed a severe defect in proliferation compared to depilation-induced early anagen control skin (19.2% Ki67+ cells/hair germ vs. 67.8% Ki67+; Figure 3b,c). These results suggest that epidermal Wnts are required for proliferation of sHG cells at the onset of anagen.
Hair follicle stem cell and dermal papilla markers are maintained in Wntless deficient hair follicles
One possibility for the observed hair cycle arrest in Wls K14cKO mice could be that HFSCs, which are required for anagen (Blanpain et al., 2004; Morris et al., 2004; Oshima et al., 2001), are lost in the absence of Wnt ligands. We analyzed hair follicles from Wls K14cKO and littermate control mice for expression of HFSC markers. At P37, HFSC markers CD34 (Trempus et al., 2003), K15 (Liu et al., 2003; Lyle et al., 1998) and S100A4 (Ito and Kizawa, 2001) were expressed in the bulge region of Wls K14cKO and control hair follicles (Figure S3a,b). Moreover, expression of K15, CD34 (Figure 3d, S3c), and TCF3 (Nguyen et al., 2006) (Figure S4) were maintained in Wls K14cKO hair follicles similar to that of littermate controls by P91, 9 weeks after induction. Quantitative analysis of K15+ and CD34+ cells on histological sections did not show a significant difference between control and Wls K14cKO telogen hair follicles at P91 (Figure S3c). Additionally, interfollicular epidermal markers of differentiation were appropriately expressed within the interfollicular epidermis of mutant mice and not within mutant hair follicles (Figure 3e,f). The DP markers alkaline phosphatase (AP) and LEF-1 were also expressed in Wls K14cKO hair follicles similar to control hair follicles (Figure 3g,h). These data show that although epidermal Wnts are required for hair growth, they are not required for preservation of HFSCs or for maintenance of other constituent hair follicle populations.
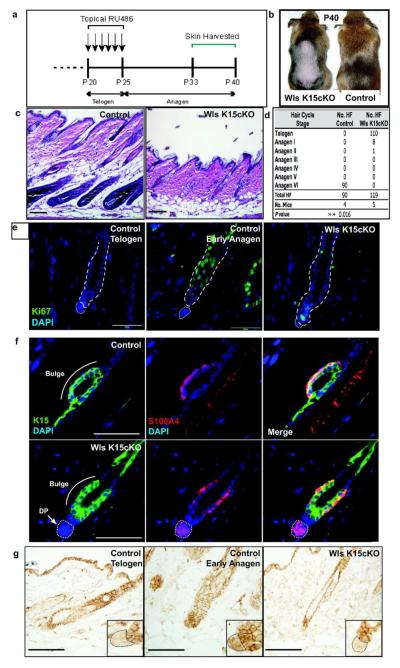
Hair follicle stem cells require Wntless for hair growth
To determine if Wls is required specifically in HFSCs to mediate anagen, we utilized the K15-CrePR1 mouse model that expresses Cre exclusively in K15+ HFSCs following topical RU486 administration (Morris et al., 2004). Dorsal skin of K15-CrePR1;Wlsfl/fl (Wls K15cKO) and littermate control mice were induced during telogen and then analyzed at P33-P40 when control hair follicles were in spontaneous anagen phase (Figure 4a). Although control mice grew a new hair coat following induction, Wls K15cKO mice failed to regrow hair in the clipped area of skin (Figure 4b). Histologically, Wls expression was significantly decreased in Wls K15cKO hair follicles compared to controls (Figure S6c). In contrast to control mice that showed progression of all hair follicles to anagen VI, 92.4% of mutant hair follicles were arrested in telogen and only 6.7% reached anagen I (Figure 4c,d). In contrast to the Wls K14cKO mice, Wls K15cKO skin did not show thickening or enlargement of the epidermis and sebaceous glands, suggesting a cell-autonomous effect of Wls depletion in epithelial cells outside of the K15+ HFSC population. Ki67 immunohistochemistry revealed a marked reduction in proliferating cells within hair follicles of mutant mice (13.5% +/− SD15.0 Ki67+ cells/hair germ) when compared to control early anagen hair follicles (69.3%+/− SD16.9 Ki67+ cells/hair germ; Figure 4e,S6a). Consistent with results obtained from Wls K14cKO mice, expression of the HFSC markers K15, S100A4, and CD34 and the DP marker, AP was maintained in Wls K15cKO hair follicles (Figure 4f, S6b).
Figure 4.
HFSCs require Wls to promote Wnt/β catenin signaling and anagen phase. (a) Mutant and control mice were shaved at P20 and RU486 was applied topically for 6 days. Skin was analyzed during anagen (P33-P40). (b) Control mice grew hair over shaved area while mutant skin remained bare by P40. (c) H&E histology of control and mutant skin at P40. (d) Corresponding morphological hair cycle stage analysis of control and mutant hair follicles. (e) Ki67 immunohistochemistry of Wls K15cKO hair follicles compared to control depilation-induced early anagen and telogen follicles (P33). (f) HFSC markers K15 and S100A4 are expressed normally within the bulge/sHG of Wls K15cKO follicles (P40). (g) β-catenin immunohistochemistry shows a lack of nuclear β-catenin in the sHG and DP of arrested mutant hair follicles, similar to control telogen follicles (white bars=50μm, black bars=100μm).
Wnt signaling is activated in the sHG and the DP during early phases of anagen (Figure 1b). To address if expression of Wls in HFSCs is required for Wnt signaling in the sHG and/or DP, we examined mutant and control hair follicles for evidence of Wnt activation as indicated by nuclear β-catenin expression (Figure 4g). Strikingly, nuclear β-catenin was not upregulated in either the sHG or DP of Wls K15cKO hair follicles. Hair follicles from Wls K14cKO mice also showed decreased number of sHG cells with nuclear β-catenin, however the number was greater and more variable than in Wls K15cKO mice (Figure S5). This suggests that Wnt ligand secretion by HFSCs is required for anagen onset and is required, directly or indirectly, for Wnt signal activation in both the sHG and DP.
Epidermal Wntless is required for adult wound-induced hair neogenesis (WIHN)
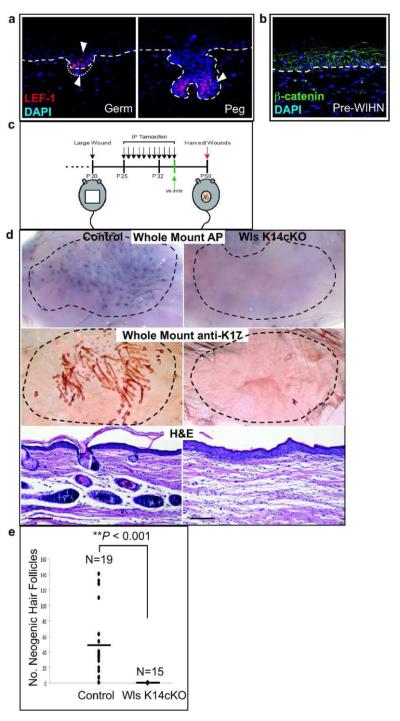
During WIHN, hair follicles regenerate de novo within the center of reepithelialized wounds (Ito et al., 2007). A previous study showed that nuclear β-catenin can be detected in both the hair follicle epithelium and dermal condensate/DP during WIHN. Consistent with this study, another transducer of Wnt signaling, LEF-1, is also first be seen in the epithelial germ and dermal condensate during the initial stages of hair neogenesis (Figure 5a). However, unlike embryonic development in which uniform upper dermal Wnt signaling precedes hair morphogenesis (Chen et al., 2012; DasGupta and Fuchs, 1999; Zhang et al., 2009) we could not detect Wnt activity in the upper dermis prior to hair germ formation by either β-catenin or LEF-1 immunohistochemistry or with a Wnt-responsive reporter (Figure 5b, data not shown).
Figure 5. Epidermal Wnt ligand secretion is required for hair follicle neogenesis following wound healing.
Epidermal Wnt ligand secretion is required for WIHN. (a) LEF-1, a marker of Wnt/β-catenin signaling, is expressed in the epithelium and mesenchyme of germ and peg stage neogenic hair follicles from wildtype mice. (b) β-catenin immunohistochemistry of WIHN in wildtype mice taken just after reepithelialization, prior to hair neogenesis. Nuclear β-catenin was not detected in either the epidermis or dermis. (c) Schematic of tamoxifen induction and wounding. (d) whole-mount AP staining of dermal wound preps from control and Wls K14cKO mice (top), whole-mount K17 staining of underside of epidermal sheets (middle), and H&E histology of wound sections (bottom; bar=100μm). Control and mutant whole-mounts were taken at same magnification. (e) Graphical distribution of number of neogenic hair follicles per wound in control and mutant mice determined by counting AP+ spots from dermis AP whole mounts (bar represents mean).
As most neogenic hair follicles are not derived from K15+ HFSCs (Ito et al., 2007), we used Wls K14cKO mice to examine the requirement for epidermal Wnt ligands in promoting WIHN (Figure 5c). Reepithelialization occurred similarly over 12-14 days in both control and mutant mice (data not shown). The DP of neogenic hair follicles were evident by AP whole-mount staining of dermis samples in healed wounds of control mice but not Wls K14cKO mice (Figure 5d, top). Anti-K17 whole-mount stains of separated epidermal sheets (middle) and H&E sections (bottom) also confirmed a lack of hair follicles in the wound epithelium of mutant mice. Although control mice showed variable numbers of neogenic AP+ DP within the wound center, only one sample from mutant mice showed one AP+ DP, while the remainder showed no neogenic hair follicles (Figure 5e). These experiments demonstrate that epidermal Wnts are required for both the hair cycle as well as adult de novo hair follicle regeneration.
Discussion
Coordinated activation of Wnt/β-catenin signaling in distinct cell types of the hair follicle is required for their synchronized growth and differentiation during anagen. Both melanocyte stem cells and HFSCs in the sHG activate Wnt signaling at anagen onset, and a previous study suggested that Wnt ligands secreted by HFSCs within the sHG are a source for the dual activation of Wnt signaling in melanocytes and HFSCs at anagen onset (Rabbani et al., 2011). Wnt signaling is also activated in DP cells to support growth of the hair follicle during anagen (Enshell-Seijffers D, 2010; Kishimoto et al., 2000; Soma et al., 2012). In this study, we sought to address the cellular source of Wnt ligands that activate Wnt signaling in the hair follicle during anagen onset. To this end, we deleted Wls specifically in the hair follicle epithelium to show that Wnt ligand secretion by HFSCs is required for activation of Wnt signaling in the sHG and DP. Furthermore, Wls-deficient hair follicles show a hair cycle arrest in telogen or early anagen phase.
Although the observed hair cycle defect is consistent with previous studies that deleted β catenin in the basal layer of the epidermis resulting in failure to enter anagen, there are the following important differences (Huelsken et al., 2001; Lowry et al., 2005). First, HFSCs of Wls-deficient hair follicles have a severe proliferation defect. By contrast, loss of β-catenin in the epidermis during telogen resulted in HFSC hyperproliferation, leading to the study’s conclusion that Wnt/β-catenin signaling is required for their quiescence (Lowry et al., 2005). Second, expression of HFSC markers is maintained for at least 2 months following induction of Wls K14cKO mice, whereas HFSCs were undetectable within 2 weeks of Cre-mediated β catenin deletion. Third, expression of interfollicular epidermal and DP markers are maintained faithfully in the absence of epithelial Wls, in contrast to results obtained from the progressive loss of epidermal β-catenin postnatally (Huelsken et al., 2001). We conclude that loss of β-catenin expression resulted in a phenotype that may be partially attributed to its function as a component of adherens junctions. It is plausible that β-catenin’s function in intercellular adhesion affects the integrity of the hair follicle and its interactions with the DP, which provides signals to regulate the quiescence and activation of HFSCs. Loss-of-function mutations in specific domains of β-catenin required for its function in adhesion and Wnt signaling in the hair follicle have not been tested.
Although our study cannot differentiate the effect of Wls in canonical or non-canonical (β-catenin-independent) Wnt signaling, the previously-established role of canonical Wnt signaling in hair growth and differentiation suggests that Wls is indispensible in HFSCs largely due to its function in canonical Wnt ligand secretion. Irrespective of Wnt ligand class, our data demonstrate that loss of canonical Wnt/β-catenin signaling in HFSCs is not sufficient to result in the extinction of HFSCs. In addition, a previous study showed that excessive Wnt ligand activity in the hair follicle resulted in loss of HFSC marker expression, suggesting that suppression of Wnt signaling in HFSCs is essential for their maintenance (Liu et al., 2007). Taken together, we hypothesize that inhibition of Wnt ligand activity may be important for maintaining HFSCs and that the compartmentalized secretion of Wnt ligands within the sHG is one mechanism to ensure the maintenance of some HFSCs (e.g. bulge cells) with each growth phase. Our observation that a few nuclear β-catenin+ and Ki67+ cells are present in the sHG of arrested mutant hair follicles may also suggest that a minimum number of cells must be activated to initiate anagen phase.
We observed that Wls is specifically upregulated in the sHG during anagen I, which correlates with Wnt ligand transcription during anagen onset (Greco et al., 2009; Rabbani et al., 2011; Reddy et al., 2001). This partly explains the preferential upregulation of Wnt signaling and proliferation of sHG cells during anagen onset. Previous studies proposed that the DP provides inductive signals to the follicular epithelium that leads to HFSC activation during anagen onset (Sun et al., 1991). Based on our study and others, it is unlikely that the DP activates HFSCs solely by providing Wnt ligands, as Wnt signaling in HFSCs is inhibited in the absence of epithelial Wnt ligand secretion. Additionally, upregulation of Wls expression in DP cells lags behind that seen within the sHG during early anagen. Nevertheless, the possibility that DP cells secrete Wnt ligands that are required for Wnt activation in HFSCs during anagen onset cannot be excluded. Reagents that specifically target the DP will be important to address this question.
We found that epithelial Wnt ligands are directly or indirectly required for Wnt signaling in the DP. Given that most canonical Wnt ligands are expressed by the follicular epithelium (Reddy et al., 2001), the DP may respond to Wnt ligands secreted by adjacent sHG and matrix cells during anagen. As DP-specific depletion of β-catenin results in an early anagen arrest (Enshell-Seijffers D, 2010), the hair cycle arrest observed in the absence of epithelial Wls may be at least partly due to defective Wnt/β-catenin signaling in the DP. Downstream factors secreted by the DP such as FGF7 and FGF10 likely play important roles to promote anagen (Enshell-Seijffers D, 2010; Greco et al., 2009). Although signals upstream of HFSC Wnt activation are still not fully understood, our data are consistent with a model in which HFSCs in the sHG respond to initiating signals from the DP (e.g. BMP inhibitors and others) to secrete Wnt ligands at anagen onset. This results in the dual activation of Wnt signaling in HFSCs themselves as well as DP cells and promotes their coordinated and reciprocal interactions throughout anagen (Figure S6e).
Finally, we demonstrate an essential role for epidermal Wnt ligands in promoting adult wound-induced de novo hair follicle regeneration. Similar to embryonic hair development, Wnt/β-catenin activation is essential to promote WIHN (Ito et al., 2007). A recent study revealed that epidermal Wls is necessary for uniform upper dermal Wnt activation that is seen prior to embryonic hair follicle development (Chen et al., 2012). Here, loss of Wls expression in the embryonic epidermis resulted in an absence of hair development, suggesting that early dermal Wnt signaling is required for hair follicle initiation. We and others found that Wnt activation is first detected in hair follicle germs and associated dermal condensates but not at an earlier stage of WIHN. Although this may reflect a limitation of detection, similar methods utilized to detect uniform upper dermal Wnt signaling prior to and during embryonic hair follicle development failed to demonstrate a similar finding during WIHN, suggesting that some differences in signaling requirements between WIHN and embryonic hair development may exist (Chen et al., 2012; DasGupta and Fuchs, 1999; Zhang et al., 2009). Regardless, this data and others suggest that Wnt signaling is required for WIHN, independent of uniform dermal Wnt activation.
Collectively, these data establish important concepts in our understanding of the regulation and maintenance of HFSCs by Wnt/β-catenin signaling and highlight the importance of Wnt ligand secretion by hair follicle epithelial cells in directing anagen onset and early events in hair regeneration. Additionally, this study sheds light onto the potential non-cell autonomous and cell-autonomous interactions that promote hair growth and holds implications for future strategies to treat hair disorders such as alopecia or hirsutism.
Materials and methods
Mice
Wntlessflox/flox (Carpenter et al., 2010) were bred to either Keratin14Cre-ER (Jackson Labs, Bar Harbor, ME) (Vasioukhin et al., 1999) or to Keratin15Cre PR1 (Jackson Labs) (Morris et al., 2004) mice. All animal procedures were done under the approval of Case Western Reserve University IACUC committee. For induction protocols see Supplemental Text.
Supplementary Material
Acknowledgments
We thank Emily Hamburg and Harry Winfield for critical review of this manuscript, Richard Lang for providing Wntlessflox/flox mice and Wntless antibody, Derrick Nau and H.W. for creative illustrations, and Wei Chin Chou for technical assistance. We are grateful to Amad Awadallah for his patience and tireless work to prepare sections. P.M. is supported by the Dermatology Foundation Dermatologist Investigator Research Fellowship and NIH T32 training grant 5T32AR007569. M.I. is supported by NIH-NIAMS grant 1R01AR059768-01A1. R.A. is
supported by NIH-NIDCR grant R01-DE01870, Basil O’Connor Award from the March of Dimes and Scleroderma Research Foundation.
Abbreviations
- HFSC
hair follicle stem cell
- sHG
secondary hair germ
- DP
dermal papilla
- ORS
outer root sheath
- Wls
Wntless
- WIHN
wound-induced hair neogenesis
- BrdU
bromodeoxyuridine
- DKK1
Dickkopf-related protein 1
- RU486
mifepristone
- FGF
fibroblast growth factor
Footnotes
Depilation and WIHN
Depilation and WIHN were done as detailed in Supplemental Text.
Conflict of Interest
The authors state no conflicts of interest.
In situ hybridization, qPCR, immunohistochemistry, proliferation assays
All assays were performed on dorsal skin as described in Supplemental Text.
References
- Andl T, Reddy ST, Gaddapara T, et al. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, et al. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, et al. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, et al. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Jarrell A, Guo C, et al. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development. 2012;139:1522–1533. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Enshell-Seijffers DLC, Kashiwagi M, Morgan BA. B-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, et al. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, et al. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ito M, Kizawa K. Expression of calcium-binding S100 proteins A4 and A6 in regions of the epithelial sac associated with the onset of hair follicle regeneration. J Invest Dermatol. 2001;116:956–963. doi: 10.1046/j.0022-202x.2001.01369.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Toyoda M, et al. Label-retaining cells in the bulge region are directed to cell death after plucking, followed by healing from the surviving hair germ. J Invest Dermatol. 2002;119:1310–1316. doi: 10.1046/j.1523-1747.2002.19644.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, et al. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, et al. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle S, Christofidou-Solomidou M, Liu Y, et al. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J Cell Sci. 1998;111(Pt 21):3179–3188. doi: 10.1242/jcs.111.21.3179. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, et al. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar S. Molecular mechanisms regulating hair follicle development. J invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Myung P, Ito M. Dissecting the bulge in hair regeneration. J Clin Invest. 2012;122:448–454. doi: 10.1172/JCI57414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, et al. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Niemann C, Owens DM, Hulsken J, et al. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, et al. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Rabbani P, Takeo M, Chou W, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Soma T, Fujiwara S, Shirakata Y, et al. Hair-inducing ability of human dermal papilla cells cultured under Wnt/beta-catenin signalling activation. Exp Dermatol. 2012;21:307–309. doi: 10.1111/j.1600-0625.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- Sun TT, Cotsarelis G, Lavker RM. Hair follicular stem cells: the bulge-activation hypothesis. J Invest Dermatol. 1991;96:77S–78S. doi: 10.1111/1523-1747.ep12471959. [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Van Mater D, Kolligs FT, Dlugosz AA, et al. Transient activation of beta -catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, et al. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tomann P, Andl T, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.