Abstract
Fatigue is a symptom associated with many disorders, is especially common in women and in older adults, and can have a huge negative influence on quality of life. Although most past research on fatigue uses human subjects instead of animal models, the use of appropriate animal models has recently begun to advance our understanding of the neurobiology of fatigue. In this review, results from animal models using immunological, developmental, or physical approaches to study fatigue are described and compared. Common across these animal models is that fatigue arises when a stimulus induces activation of microglia and/or increased cytokines and chemokines in the brain. Neurobiological studies implicate structures in the ascending arousal system, sleep executive control areas, and areas important in reward. In addition, the suprachiasmatic nucleus clearly plays an important role in homeostatic regulation of the neural network mediating fatigue. This nucleus responds to cytokines, shows decreased amplitude firing rate output in models of fatigue, and responds to exercise, one of our few treatments for fatigue. This is a young field but very important as the symptom of fatigue is common across many disorders and we do not have effective treatments.
Keywords: fatigue, cytokine, suprachiasmatic, circadian, animal model
Introduction
Fatigue is one of the most common complaints that brings patients to see their clinician in part because feeling unusually fatigued can dramatically impact quality of life. "Feeling tired for no reason", a common report in people suffering from fatigue (Kirsh et al., 2001), leads to reduced ability to work, to enjoy life, and to sleep well. Fatigue is a symptom associated with illness, depression, and aging, and is reported as a primary symptom in multiple sclerosis and Parkinson’s disease, post-stroke, and post-poliomyelitis. Fatigue is an important symptom of frailty in aged populations (Fried et al., 2001) and predicts functional limitation (Avlund et al., 2003a), disability (Avlund et al., 2003b) and mortality (Hardy and Studenski, 2008, Moreh et al., 2010). In cancer patients fatigue is often experienced pre-diagnosis, can be a result from treatments, and can be a lasting problem even following cure (Wang, 2011). A traumatic brain injury is often followed by lasting fatigue and sleep disorders (Cantor et al., 2008). One would think that such an important and widely experienced symptom would be extensively researched, since any insight into biological mechanisms could potentially lead to a treatment applicable to many populations. The difficulty in defining fatigue and in developing animal models for the study of fatigue has impeded progress in this field.
Researchers have found it hard to agree on a definition of fatigue. There are more than 250 self report measurement devices for assessing fatigue in human populations in the literature, about 150 of these measurement scales apparently only used once (Hjollund et al., 2007). Recent efforts to draw together a consensus definition and method for assessment of fatigue promise to help advance this field (Barsevick et al., 2010). Even more importantly, several animal models have been developed with common features, leading us to believe we are coming to a consensus on the objective manifestation of a state of fatigue in a laboratory animal. To make rapid progress in our understanding of this distressing symptom, animal models will allow controlled studies targeted to mechanism and/or therapy.
In this review I will describe these animal models as well as a new model my laboratory is developing. Fatigue may be a subjectively defined state, but objective correlates can be measured. Research has already produced some lines of evidence to help shape our hypotheses of neurobiological substrates underlying fatigue, and I will review those data, with particular emphasis on evidence for the importance of the circadian system in the experience of fatigue.
1. The human experience of fatigue
1.1 Defining fatigue
It is critical for this work that we can define the state of fatigue, and yet that is a difficult task. Researchers need to distinguish the way a healthy person experiences fatigue from the cases where fatigue is a disabling symptom of poor health. It is important to listen carefully to the ways people describe their experience of pathological fatigue, and to distinguish it from non-pathological fatigue. Non-pathological fatigue would be the experience of being tired after exercise, with energy restored after rest. Pathological fatigue is described as having seven primary characteristics (Barsevick et al., 2010). It is subjective, and unusual (not proportional to prior activity and not relieved by rest). Physical sensations range from lassitude to exhaustion, and the fatigue has a negative impact on function (decreased capacity for work, poor sleep quality, withdrawal from activities). There is decreased cognitive ability and an unpredictable temporal course (the fatigue can be either chronic or acute). The negative emotions associated with fatigue include helplessness, vulnerability, impatience, anxiety, and emotional numbness (Barsevick et al., 2010). A definition of cancer-related fatigue proposed by the working group ASCPRO (Assessing Symptoms of Cancer Using Patient-Reported Outcomes) captures these attributes in defining fatigue as the perception of unusual tiredness that varies in pattern or severity and has a negative impact on ability to function (Barsecick et al., 2010). It is also important to distinguish fatigue from depression, sleepiness, sickness, apathy, or other closely related or often co-morbid states. Optimal would be a definition that allowed objective measures of the state, although we might be content at this point if we can simply agree on a definition allowing consistent self-report measures in human populations; unfortunately there is at this moment no agreement. Current recommendations from systematic reviews suggest that the specific measurement scale to use for assessment of fatigue should be selected for the specific clinical population being studied (Elbers et al., 2011). In many cases a multidimensional scale is used, allowing a multifaceted assessment that can capture the physical, affective and cognitive aspects of this symptom. It may seem better practice to ask people to report only on their current state of fatigue to avoid retrospective bias, but this can miss the variable temporal course, so scales generally ask for a judgment on each item for some set reporting period (e.g., over the past week) which varies across scales. Thus, with no agreement on the measurement device it is difficult to compare studies assessing the rate of occurrence of the state, as well as the impact of treatments, etc. While it is not the purpose of this review to sort this out, the current state of affairs is impeding progress in the field and it would be better if researchers could reach some consensus on measurement scales for assessing fatigue in human populations.
This review is focused on acute fatigue, with attention to central nervous system correlates of fatigue. Researchers have differentiated "peripheral fatigue" and "central fatigue" (Cantor, 2010), although some question if this is a useful distinction (Gerber, 2010). Peripheral fatigue presumably arises from muscle fatigue and fatigability while central fatigue is thought to reflect central nervous system processes. The difficulty in making a clear distinction arises from studies showing a role for the central nervous system in fatigue initially thought to arise from the periphery. Both mental fatigue (increased cognitive effort, difficulty sustaining attention) and physical fatigue (failing to initiate physical acts) can arise from central nervous system derived fatigue (Chaudhuri and Behan, 2000). The focus in this paper is on acute fatigue of the sort that might be triggered by a viral infection or radiation therapy, and that may last well after the triggering event. Chronic fatigue syndrome is more than simply the experience of fatigue in that it is defined by debilitating fatigue lasting 6 months or longer, along with other symptoms such as cognitive dysfunction, sleep disturbance, myalgia, arthralgia, headache, gastrointestinal upset, sore throat and painful lymph nodes (Christley et al., 2012).
1.2. Treatments for fatigue
Clinical assessments of fatigue must determine severity and then how much the fatigue interferes with quality of life, including assessment of effect on social function, cognition, mood, and physical function (Wang, 2011). Potential causes such as anemia, infection, malnutrition, chronic pain, centrally acting drugs, etc. should be assessed. Fatigue may be straightforward to treat if it can be ascribed to known causes. For example, fatigue might be ascribed to anemia, malnutrition, hypothyroidism, infection, etc. Sleep disorders are linked to self-reports of fatigue, and self-reports of fatigue are associated with both objective and subjective measures of poor sleep (Liu et al., 2012). Clinicians will investigate to determine if any likely causal factors can be identified and if so, if these factors lead to a treatment path (Horneber et al., 2012).
Yet many cases of fatigue are not easily treatable and in fact patients often assume that the clinician will have no treatment and therefore they do not even bother to mention the symptom of fatigue. Another complication in assessment arises when patients adapt to an altered level of fatigue, defining that as the "new normal" and therefore begin to under-report fatigue ("response shifts"; Wang, 2011).
Treatments are limited, although exercise and cognitive behavior therapy have some benefits (Price et al., 2008), as can psychostimulants (Horneber et al., 2012). Studies suggest lasting benefits from aerobic exercise (averaging 75% of maximum heart rate for 3 h/week), and possibly also benefits from anaerobic exercise and relaxation training (McNeely et al., 2006). Cognitive behavioral therapy was shown to be effective in treatment of fatigue in patients with multiple sclerosis (van Kessel et al., 2008). It appears that the critical component of this therapy is the success in changing negative cognitions about fatigue, but instead perceiving it as something that is time-limited and has less severe consequences (Knoop et al., 2012). Cognitive behavioral therapy delivered via the internet was recently shown to be more effective than usual care for adolescents with chronic fatigue syndrome (Nijhof et al., 2012). Combining cognitive therapy with exercise is more beneficial than cognitive therapy alone for post-stroke fatigue (Zedlitz et al., 2012). Modafinil was shown to be effective in treating patients with severe cancer-related fatigue (Jean-Pierre et al., 2010), and HIV-related fatigue (Rabkin et al., 2011) but was not effective for fatigue associated with multiple sclerosis (Kos et al., 2008). Methylphenidate can be helpful in cases of cancer-related fatigue (Horneber et al., 2012). A small trial indicated some benefit from light therapy for fatigue in breast cancer patients (Ancoli-Israel et al., 2012). Serotonergic antidepressant drugs have in several studies been reported to be ineffective to reduce symptoms of fatigue even when they are effective for symptoms of depression (Morrow et al., 2005). In support of this, people with hepatitis C treated with the cytokine IFN-α show high incidence of fatigue and depression, with the depressive symptoms responding to serotonergic antidepressants but the fatigue symptoms being resistant to this treatment (Capuron and Miller, 2011).
There are several "take-home" messages from this brief survey of current treatments. First, we have some causes of fatigue that we can treat, such as anemia. In building animal models we should screen for these causes in the same way we would with a patient presenting with fatigue. Second, there is a scarcity of treatments in many cases of fatigue, with one of the most effective, exercise, being difficult to administer to a patient suffering extreme fatigue. Third, some treatments may help us to better understand the underlying mechanisms generating pathological fatigue. For example, research on the success of cognitive behavioral therapy in treatment of fatigue suggests that cognitions can worsen fatigue. This will be a factor important in the human population that may not be able to modeled in laboratory animals. On the other hand, lack of success of anti-depressants may point to separable neural substrates for fatigue and depression, whereas the limited success of stimulants may focus our research on the role of neural pathways activated by these pharmacological agents.
2. Animal models of fatigue
An ideal animal model of fatigue would encompass as many attributes of the human experience of fatigue as possible (strong face validity), while remaining specific to fatigue. Using the seven unique characteristics of fatigue identified from a literature review of conceptual definitions as summarizing in Barsevick et al. (2010), we can consider in the broadest sense the range of measures an animal model might include (see Table 1). To identify neural substrates underlying fatigue we would like to have an animal model that as nearly as possible involves only the symptom of fatigue, without other closely associated states.
Table 1.
Characteristics of fatigue in humans common to many definitions (from Barsevick et al., 2010) and suggestions of how these might be translated to an ideal animal model of fatigue.
| Characteristic | Descriptors from studies of humans |
Possible translation to rodent model |
|---|---|---|
| Subjective | assessed by self-report | Unable to translate this to rodent model |
| Physical sensation | "exhaustion, decreased energy, weakness, malaise, tiredness, lassitude" | Decreased spontaneous wheel-running activity, decreased burrowing, changes in spontaneous locomotor activity |
| Unusual | "unrelieved by rest", "not proportional to activity, unpredictable" | Symptoms that are different from those of control animals |
| Impact on functioning | "decreased capacity for work, decreased quality of life, difficulty completing tasks, poor sleep quality, withdrawal from activities, debilitation" | Altered sleep, shortened duration of circadian active phase, decreased social interactions |
| Unpleasant emotions | "helplessness, vulnerability, distress, reactivity, impatience, anxiety, emotional numbness, unpleasant experience, emotional lability" | Altered performance on learned helplessness tasks, changes in tests of anxiety such as the elevated plus maze |
| Decreased cognitive ability | "decreased attention, decreased concentration, decreased motivation, memory deficits, decreased mental capacity, decreased capacity for mental work" | Deficits in tests of attention, cognition, motivation, memory |
| Temporal variability | "pervasive, chronic, acute, persistent, episodic" | Unrestrictive in terms of the temporal course |
Second, an ideal animal model would also be induced by a stimulus that can induce fatigue in humans (strong construct validity). Research on laboratory animals using reliable measures of fatigue should allow us to determine further factors that can cause the experience of fatigue.
Third, an ideal animal model would involve changes in behavior, cognition, or physiology that are reversed by the same treatments that can reverse fatigue in people (strong predictive validity). Of course we also hope that a strong animal model will allow us to discover novel treatments for the relief of fatigue, but we validate the animal model first using what few treatments are currently clinically employed.
We are in the early stages of establishing such a comprehensive animal model. Most of the animal research reviewed below includes measures attempting to capture the physical sensations associated with fatigue. The emotional and cognitive aspects of the human experience of fatigue should be included in future research developing these models. Most current models are based on an understanding of fatigue as arising from a pro-inflammatory state. Very little current work can claim predictive validity for their animal model of fatigue.
2.1 A variety of animal models
Although it has been suggested that we could develop 4 types of animal models of fatigue (Katafuchi et al., 2006), targeting physical fatigue (forced exercise, swimming), mental fatigue, environmental fatigue (heat exposure), or immunologically induced fatigue, most studies have used the latter, immunologically induced fatigue. Studies using forced exercise and swimming run into difficulty separating the effects of the stress of the treatment and the process of fatigue. For example, one group has studied rats subjected to electric shock and either restraint stress or cold water swim on a daily basis for 23 days as a model for chronic fatigue syndrome (Zou et al., 2010). This model is based in a conceptual understanding of chronic fatigue syndrome as arising from stress, but might be better thought of as a model for the study of effects of stress. Similarly involving stress, a model of fatigue based on chronic sleep deprivation uses rats housed in a cage filled with water to about 2 cm (Tanaka et al., 2003). On a perhaps milder note, one study measured grooming and rearing after a 5 min daily swim as a plausible model of post-exercise fatigue (Chao et al., 1992).
Because chronic fatigue syndrome often starts after a flulike episode, suggesting a viral cause, and other viruses are associated with lasting fatigue (eg, mononucleosis), some researchers have developed animal models of fatigue using a virus. What may be one of the most promising current models of fatigue involves systemic administration of polyinosinic: polycytidylic acid (poly I:C), a synthetic analog of double-stranded RNA that mimics viral infection. This treatment in rats induces a decrease in spontaneous wheel running persisting about a week (see figure 1), and then gradually recovering over the next week (Katafuchi et al., 2003). The acute response, such as a rise in body temperature, lasts only 24 h. Strikingly, these rats do not show reduced levels of activity or exploration in the open field 7 d after injection, even when voluntary wheel-running is greatly reduced. This suggests that they are not impaired in motor activity in general or experiencing joint or muscle pain, and indicates a possible effect more centrally on motivated activity. Changing to an icv route of administration of poly I:C induces reductions in locomotor activity lasting at least 28 days (Patro et al., 2010).
Figure 1.
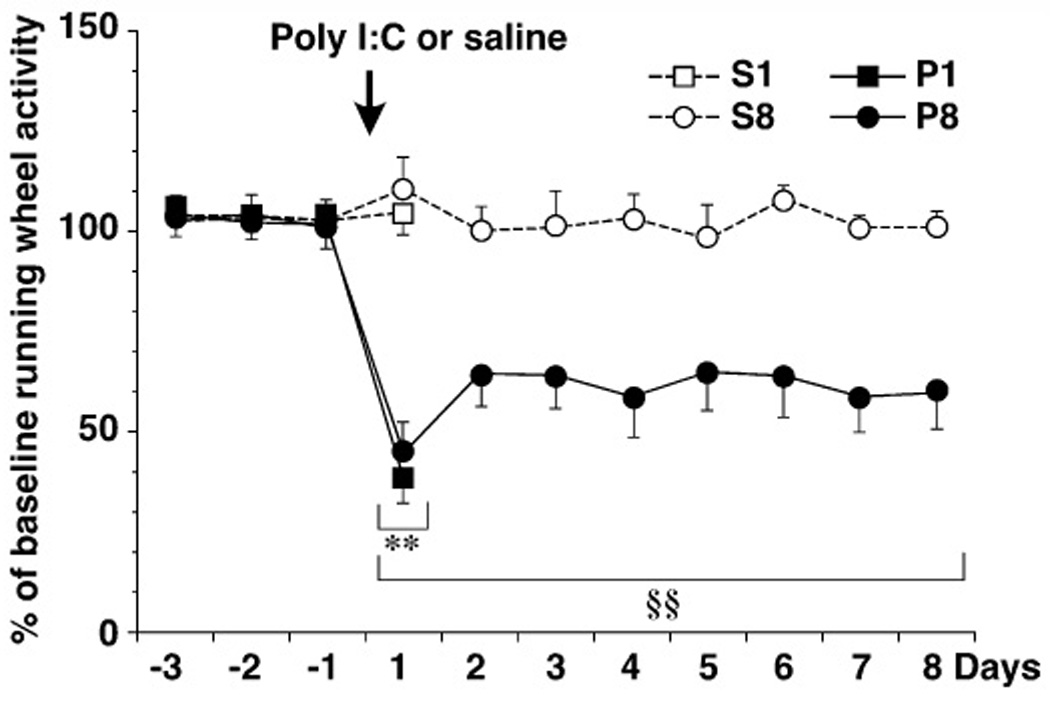
Suppression of spontaneous wheel-running activity following poly I:C. Male Wistar rats were administered poly I:C (P; 3mg/kg) or saline (S) and killed on day 1 or day 8 following injection. Total daily wheel-running activity was expressed as a percentage of baseline. (N=5/group; from Katafuchi et al., 2005).
Parasitic infections have also been used to model immunologically-induced fatigue in rodents. Both Cryptosporidium parvum and Toxoplasma gondii infections cause a drop in wheel running in mice lasting several weeks (Chao et al., 1992). Rats infected with the parasite Trypanosoma brucei, associated in humans with human African trypanosomiasis or "sleeping sickness", show reduced amplitude circadian activity rhythms, mirroring effects in humans infected with this parasite who show diurnal somnolence and nocturnal insomnia (Kristensson et al., 2010, Lundkvist et al., 2010). Activation of astrocytes and microglia as well as increased levels of pro-inflammatory cytokines and the chemokine CXCL10 are observed in the brains of rats infected with T.brucei (Bentivoglio et al., 2011).
Mice treated with killed Brucella abortus bacterial antigen showed a long lasting (e.g., several week) suppression in wheel-running, explained by an abbreviated duration of activity rather than lasting changes in level of peak activity (Ottenweller et al., 1998). The suppression of wheel-running activity lasted longer than signs of poor grooming. The inducing injection appears to lead to a short-term illness that is followed by a long-lasting state of fatigue, accompanied by changes in immunological markers (Ottenweller et al., 1998), mirroring some of the most puzzling cases in humans where the inducing disease seems to be in abeyance yet fatigue remains as a persistent symptom. On the other hand, a recent report indicates that mice injected with B. abortus antigen can develop anemia over 10 days that recovers in 3–5 weeks, also showing a persistent increase in IL-6 (Sasu et al., 2010). It is critical for future use of this model that the anemia be corrected and then the mice tested to see if the suppression of activity is due to anemia or is separable from this. This model has been extended in mice given 6 injections of killed B. abortus antigen over 12 weeks, with the animals then showing suppression of wheel-running lasting at least 7 weeks after the last of 6 injections (Moriya et al., 2011). This modification offers an advantage of a longer stable state following induction, allowing tests of treatments that might require some time for beneficial action. It is hard on the animals; approximately one-third of the mice die during the period of repeated injections (Chen et al., 2008; Moriya et al., 2011).
Injections of lipopolysaccaride (LPS), a bacterial endotoxin that can stimulate an innate immune response, has been used to model “sickness behavior” (Dantzer, 2009), and fatigue is one symptom among many. Acute sickness behavior lasts a day or so, but a central response induced by peripheral stimuli can lead to proinflammatory cytokines being elevated for months. In one study mice given a single injection of LPS showed increased TNFα in the brain for 10 months, even when serum levels had returned to normal by 9 h post-injection (Qin et al., 2007). This response was not observed in mice without the TNF receptor, offering a control condition that should be useful in studies focused on the role of central cytokines in fatigue. Another study showed activation of cortical microglia and altered spine density up to one month post-LPS injection (Kondo et al., 2011).
Other researchers have studied very low doses of LPS that can alter behavior without inducing fever or signs of sickness. A sensitive behavior assay is that of burrowing; mice will reliably empty a tube filled with material placed in their cage, but mice treated with subpyrogenic doses of LPS show dramatic reductions in this activity (Teeling et al., 2007). This effect on burrowing behavior could be blocked by pretreatment with a COX inhibitor, indomethacin, suggesting a role for prostaglandins in mediating the effect.
Voluntary wheel-running is reduced following LPS administration and this effect depends in part on peripherally increased IL-6 (Harden et al., 2011). Reduced social interaction observed following LPS injection was shown to be due to IL-1β acting centrally in studies using icv infusion of an antagonist to IL-1β (Konsman et al., 2008). Similarly, reduced wheel-running in rats treated with LPS was reversed by a capsase-1 inhibitor icv, preventing cleavage of pro-IL-1β to the active form, again implicating central IL1β in behavioral manifestation of fatigue (Harden et al., 2011).
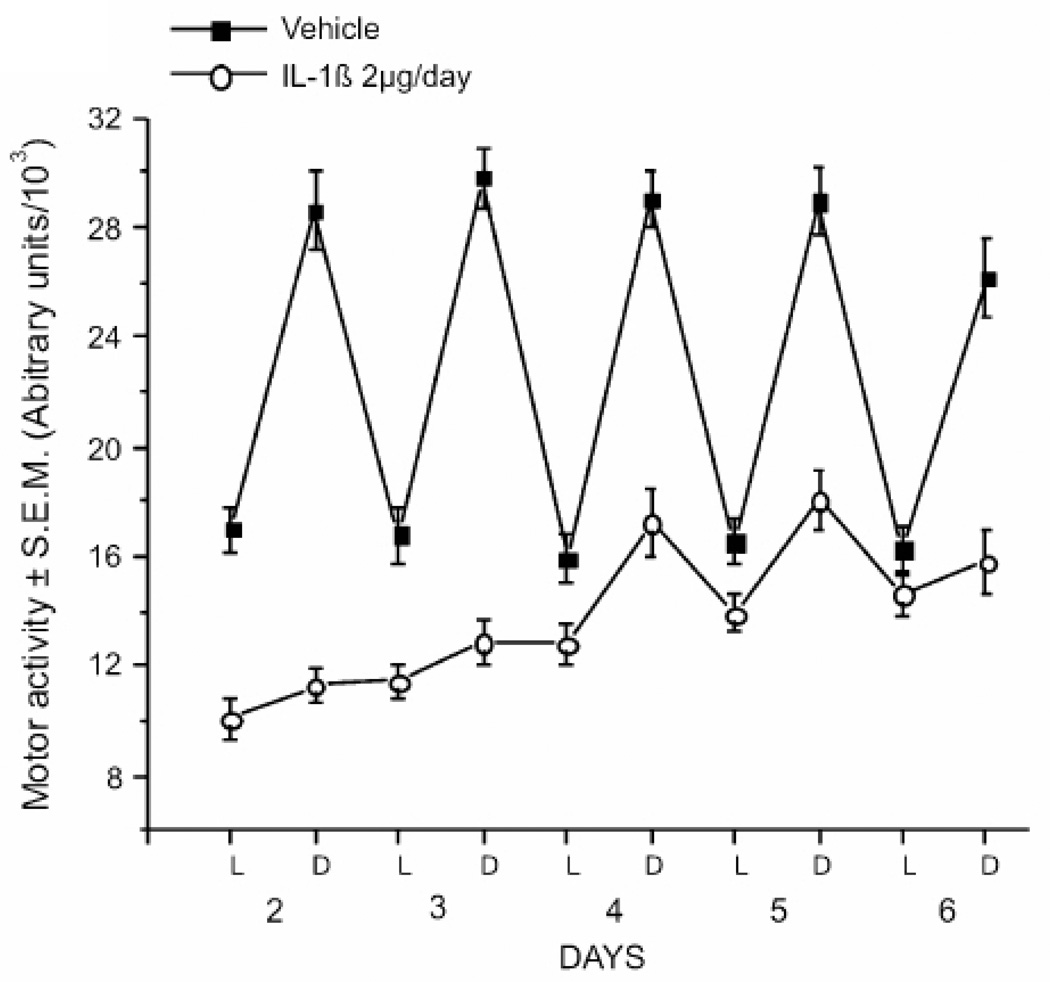
In my laboratory we have begun to study rodents treated with IL-1β as a model of fatigue. Prior work with clinical samples has shown associations between fatigue symptoms and more stable downstream indicators of IL-1β activity (e.g. IL-1 receptor antagonist; Bower et al., 2009, Schubert et al., 2007). Increased levels of IL-1 receptor antagonist are associated with increased fatigue in women with breast cancer independent of associations with sleep quality (Liu et al., 2012). Polymorphisms in the IL-1β gene predict fatigue in a small sample of breast cancer survivors (Collado-Hidalgo et al., 2008). IL-1β can induce a cascade of cytokine activity whereby other cytokines are increased in the brain and in the periphery (Anisman et al., 2008). Increased IL-1β is predicted to increase other pro-inflammatory cytokines, TNF-α and IL-6 and IL-18, as well as levels of IL-1 receptor antagonist (Bandeen-Roche et al., 2009, Morley and Baumgartner, 2004). IL-1β leads to increased but fragmented NREM sleep, and brain levels show diurnal changes with peak levels in the sleep phase (Imeri and Opp, 2009, Ray et al., 2008). It has been demonstrated that mice administered IL-1β over 5 days show reduced general locomotor activity and decreased circadian variation in activity (Anisman et al., 2008)(see figure 2).
Figure 2.
Infusion of IL-1β suppresses motor activity in mice. Male CD-1 mice were given either vehicle or IL-1β (2µg/day) via osmotic mini-pump for 7 days (N=16/group) and home cage motor activity was measured. Activity is shown as mean ± SEM in 12 h epochs corresponding to the light and dark portions of the LD cycle. From Anisman et al., 2008.
Shorter duration (circa one day) effects on locomotor activity are seen when TNF-α is administered subcutaneously (Cavadini et al., 2007) or CD40 antibody is administered as a model for autoimmune disorders (Taraborrelli et al., 2011). The response to CD40 antibody was not observed in mice without TNF or the receptor TNFR1 indicating that this response is more dependent on TNF than the other pro-inflammatory cytokines tested, IL-6 or IL-1β.
Aging and neurodegenerative disorders could also be proposed as animal models of fatigue, since they can involve the symptom of fatigue. They also share some level of centrally increased cytokines and chemokines as well as activated microglia (Corona et al., 2012, Perry, 2010). The peak amplitude and coherence of the wheel-running rhythm declines as mice age, an effect seen in both wildtype and mice over-expressing alpha synuclein, a model for Parkinson's disease. Over the age range studied, alpha synuclein over-expressing mice showed decreased wheel-running with increased number of activity bouts as compared to wildtype controls (Kudo et al., 2011a).
Researchers are just starting to develop models that will help us to better understand why some people show vulnerability to developing fatigue and others are more resilient. For example, mice previously exposed to murine gammaherpesvirus 68 showed prolonged signs of fatigue following LPS injection, with reduced wheel running for 5 days following LPS treatment in the previously infected group, a much more prolonged response than was seen in the group given LPS without a pre-treatment (Olivadoti et al., 2011).
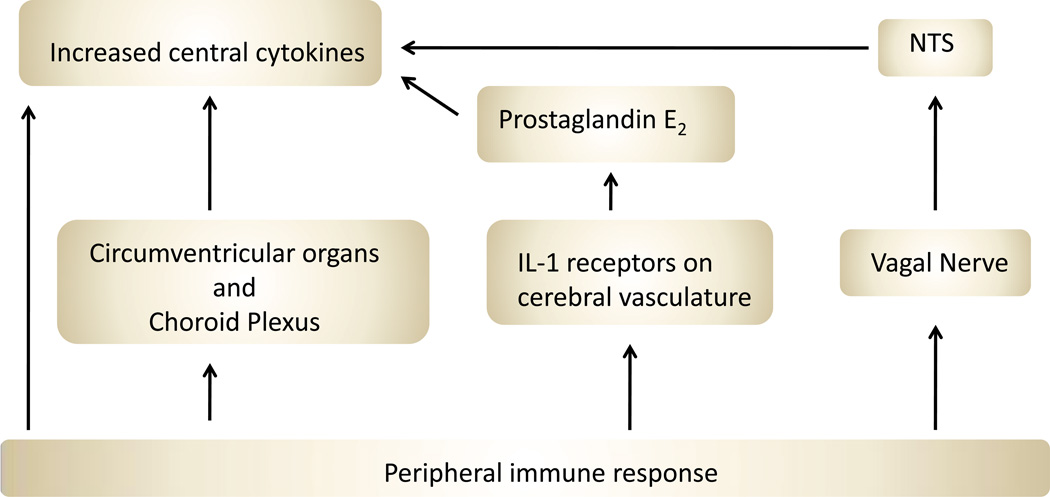
What is common across many of these models is that fatigue appears to arise when some stimulus induces increased proinflammatory cytokines in the brain and/or activated microglia and increased expression of chemokines. A peripheral immune challenge is thought to impact the central nervous system through several possible routes: by direct cytokine transport across the blood-brain barrier, by stimulation of circumventricular organs and choroid plexus cells to produce cytokines, by vagal nerve stimulation, or by direct activation of brain vasculature (see Figure 3) (Dantzer et al., 2008). Cytokines are also synthesized and released by neurons and glial cells of the CNS. A cytokine response to a treatment such as an injection of poly I:C can be measured both peripherally and centrally and is complex in terms of the number of cytokines increased and the time course (Cunningham et al., 2007).
Figure 3.
Schematic of peripheral immune challenge routes to brain. Peripheral signals of inflammation can take 4 main routes to influence the nervous system. Some cytokines are transported directly across the blood-brain barrier. Others stimulate cells in the circumventricular organs and choroid plexus to produce cytokines that diffuse into the brain. IL-1 can stimulate receptors on macrophages and endothelial cells associated with the cerebral vasculature, and these stimulate the release of prostaglandin E2. Finally, peripheral signals can directly activate the vagal nerve, which can alter brain response via a relay in the nucleus of the solitary tract (NTS).
2.2 Suggestions for further validation and development of animal models of fatigue
We can evaluate these animal models in terms of face validity (do they mirror the symptoms of this state in humans?), construct validity (do the physiological mechanisms underlying the model match those thought to underlie the human disorder?), and predictive validity (do the treatments that reverse the symptom in humans also reverse the signs in the animal model?). The models based on reduced wheel-running activity have good face validity in that the measure of reduced voluntary physical exercise is reported in people with fatigue. This behavior is also relatively easy to measure and quantify. In some cases researchers find wheel-running decreased but other, less effortful, motor behaviors unchanged, and this seems to be an especially attractive model of fatigue in terms of face validity. We can definitely do more to increase face validity, as discussed below. Many of the above animal models have construct validity if we accept that fatigue is the result of inflammation leading to central increases in cytokines, chemokines and microglia activation. Future work can extend this by including factors that are linked to increased susceptibility to fatigue, such as gender and age. Predictive validity relies on having effective treatments; currently modafinil and exercise offer the only somewhat supported possibilities in clinical populations. Little progress has been made as yet in testing treatments and their ability to reverse effects in the varied animal models.
We have several promising animal models to study fatigue, but we could develop these further to increase their validity. First, it would be useful to include multiple measures to better characterize effects. Particularly useful are measures of spontaneous effortful activity, such as voluntary wheel-running or burrowing (Deacon, 2009). Studies should also include less effortful locomotor activity that should be preserved at more normal levels (Katafuchi et al., 2003, Olivadoti et al., 2011). It is important to note that wheel-running is not simply another measure of locomotor activity. For example, mice bred for high levels of wheel-running do not show changes in locomotor activity in an open-field (Bronikowski et al., 2001) and mice bred for high levels of open field activity do not show changes in voluntary wheel-running (DeFries et al., 1970). Wheel running is self-reinforcing, and many species have demonstrated they are motivated to use a running wheel (Sherwin, 1998). It is not, as some have assumed, a behavior chosen only because of the degraded laboratory environment; animals in complex or seminatural environments will still choose to spend considerable time in a running wheel (Sherwin, 1998). The rewarding and motivated aspect of voluntary wheel running distinguishes this from open field activity (thought to reflect response to novelty and exploration), forced treadmill running, or simply home cage activity. Burrowing, a behavior measured in fewer studies, may be a similarly rewarding motivated activity.
Clinical reports of fatigue include negative impacts on day-to-day functioning, unpleasant emotions, and decreased cognitive abilities (see Table 1). This is a relatively unexplored area in the animal models we have reviewed. While all patients do not experience all symptoms, we should still be making an effort to include measures in our rodent studies that could capture some of these aspects of the human experience of fatigue.
Future research should carefully distinguish the specificity of these models. For example, measures of sucrose preference, a common method to measure anhedonia, could suggest that a drop in effortful activity does not arise from anhedonia. Measures of diurnal rhythms in body temperature and daily distribution of sleep could be used to assess fever or sleep disruption (e.g. Olivadoti et al., 2011). It would be helpful to clearly distinguish sleepiness from fatigue. Sleep deprivation leads to increased theta power in the EEG (Vyazovskiy and Tobler, 2005) and periods of reduced neural activity in cortical areas during waking (Vyazovskiy et al., 2011). It would be interesting to investigate if immunologically-induced fatigue or age-related fatigue are associated with similar neurophysiological changes as seen with sleepiness. In other future research we could explore the possibility of other potential factors such as allodynia, hyperalgesia, etc. mediating changes in behavior. Within a review on assessment of fatigue in clinical populations the authors note "The investigator should also measure other symptoms that could be confounded with cancer-related fatigue, such as sleep disturbance; emotional distress; and/or depression, anorexia, and anemia."(Barsevick et al., 2010). This is also true for animal models of fatigue. As much as possible, investigators should try to assess the presence of each of these potential confounding variables. An exemplary paper (Ray et al., 2011) includes hematology and serum cytokine analysis at several time points post-treatment, as well as assessment of sleep disturbances, food intake, and neurotoxicity screens within the context of an experiment comparing two chemotherapeutic agents for effects on running-wheel activity.
Future work on this topic should consider effects of both gender and age, and potential interactions between these important variables. Women are more likely to report having fatigue, often report more severe fatigue (Torres-Harding and Jason, 2005), and are more likely to seek medical help for their fatigue (Cope, 1992). Effects of aging on the immune system are gender-specific (Goetzl et al., 2010). Self-reported fatigue is twice as prevalent in older women than in men (Vestergaard et al., 2009). Permeability of the blood-brain barrier may depend on age and gender (Bake and Sohrabji, 2004, Bake et al., 2009) possibly leading to changes in neural response to peripheral inflammation (Dantzer, 2009). With age comes increased risk for persistent fatigue (Clark et al., 1995). Aging is also associated with chronic low-grade inflammation and increased levels of pro-inflammatory cytokines (Ferrucci et al., 2005). Inflammation can lead to fatigue that is experienced as more severe in older patients (Bautmans et al., 2010). A topic that has not yet been explored is whether the etiology of fatigue differs with gender and/or age. A short-term inflammatory response has greater effects on activity levels and brain IL-1 β in aged (20–24 mo) mice (Abraham and Johnson, 2009, Godbout et al., 2008). On the other hand, one study of male rats with IL-1 β delivered directly into the brain icv reports that aged rats show diminished NREM sleep response even when the induced fever was equivalent to that seen in younger rats (Imeri et al., 2004). Exploration of fatigue beyond young adult male rats and mice, the animal models dominating this and other fields (Beery and Zucker, 2011), could help us to determine if the research we are conducting might be broadly relevant across multiple species, ages and genders.
3. The neurobiology of fatigue
3.1 The neurobiology of voluntary wheel-running
Running wheel activity likely arises from the activity of multiple neural networks. Recent research demonstrates a role for neurons in the ventral tegmental area (VTA). In vivo recordings from the VTA while mice ran in wheels demonstrated that non-dopaminergic VTA neurons fire in a rhythmic pattern closely correlated with the running speed as well as the cyclicity of limb movements during running (Wang and Tsien, 2011). Putative dopaminergic VTA neurons also showed firing correlated with wheel use, but it was less strongly correlated with the behavior, and more closely linked to the beginning and end of the voluntary wheel running (Wang and Tsien, 2011). Researchers have demonstrated a genetic basis for running wheel activity, in part by experiments involving selective breeding of rats based on wheel revolutions, apparently selecting for speed of running rather than duration (Knab and Lightfoot, 2010). The dopamine D1 receptor in the nucleus accumbens (NAc), a major target for VTA neurons, plays a role in rats showing high levels of voluntary wheel use (Roberts et al., 2011). The nucleus accumbens and dorsal striatum show high levels of dopamine and dopamine metabolites in mice bred for high rates of wheel-running even when mice were not allowed wheel access (Mathes et al., 2010). Polymorphisms in dopamine receptor genes have been linked to levels of physical exercise in humans (Knab and Lightfoot, 2010) supporting a link between dopamine and wheel running. Other brain areas implicated in the neurobiology of wheel-running by studies using Fos staining were the dentate gyrus of the hippocampus, the caudate-putamen, the prefrontal, medial frontal and sensory cortex, and the lateral hypothalamus (Rhodes et al., 2003, Rhodes et al., 2005).
Other neurotransmitters may also play a role. Several studies indicate the endocannabinoid system mediates voluntary physical activity in part (De Chiara et al., 2010, Dubreucq et al., 2010). In addition, orexin A can induce spontaneous locomotor activity (Teske et al., 2008, Teske et al., 2010). Humans with narcolepsy, thought to be due to loss of brain orexin, show high rates of fatigue, separable from the excessive daytime sleepiness that is also seen with this disorder (Droogleever Fortuyn et al., 2012), suggesting orexin might play a role in the neurobiology of fatigue.
Studies using animal models of fatigue have implicated the hippocampus, an area where Fos induction was highly correlated with wheel-running (Rhodes et al., 2005). Rats given poly I:C icv showed microglial activation in the hippocampus (Patro et al., 2010). Aged mice treated with LPS showed disrupted memory consolidation and increased IL-1β expression in hippocampus and cortex (Tarr et al., 2011). Infusion of IL-1β increased cytokine mRNA expression in the hippocampus and prefrontal cortex of mice (Anisman et al., 2008). Mice treated with repeated injections of killed B. abortus antigen show hippocampal atrophy associated with reduced expression of brain-derived neurotrophic factor (BDNF) and increased expression of acetylated p53 protein in the hippocampus (Moriya et al., 2011). Decreased BrdU labeling and increased TUNEL labeling in these mice indicated reduced hippocampal neurogenesis and increased apoptosis; however, these effects on the hippocampus might be related to the stress of the repeated injections and immunological activation. Infusions of BDNF into the hippocampus did not alter level of locomotor activity in an open field (Shirayama et al., 2002).
Brain imaging of fatigued human subjects has provided little agreement to date of brain regions mediating the experience of fatigue, but striatum and cortical areas often are highlighted (DeLuca et al., 2009). Parkinson's patients with fatigue showed reduced serotonin transporter binding in caudate, putamen, ventral striatum, and thalamus when compared to similar patients without the symptom of fatigue (Pavese et al., 2010). Increased lesion load is correlated with fatigue in multiple sclerosis, with studies suggesting a functional cortical reorganization in response to the disease might underlie fatigue (Kos et al., 2008). Signs of atrophy in the striatum, thalamus, frontal and parietal cortex were observed in a sample of multiple sclerosis patients with fatigue (Calabrese et al., 2010). Damage to the basal ganglia and internal capsule was predictive of post-stroke fatigue (Tang et al., 2010). Interestingly, a new study indicates that lesions to the basal ganglia (striatum, caudate/putamen, globus pallidus) change level of wakefulness and fragmented sleep in rats (Qiu et al., 2010), supporting the suggestion from human brain imaging studies that this brain region might play an important role in fatigue. Ventral striatum contains the nucleus accumbens, discussed above in terms of mediating rewarding aspects of voluntary exercise.
3.2 The neurobiological correlates of fatigue: arousal, sleep/wake, reward systems
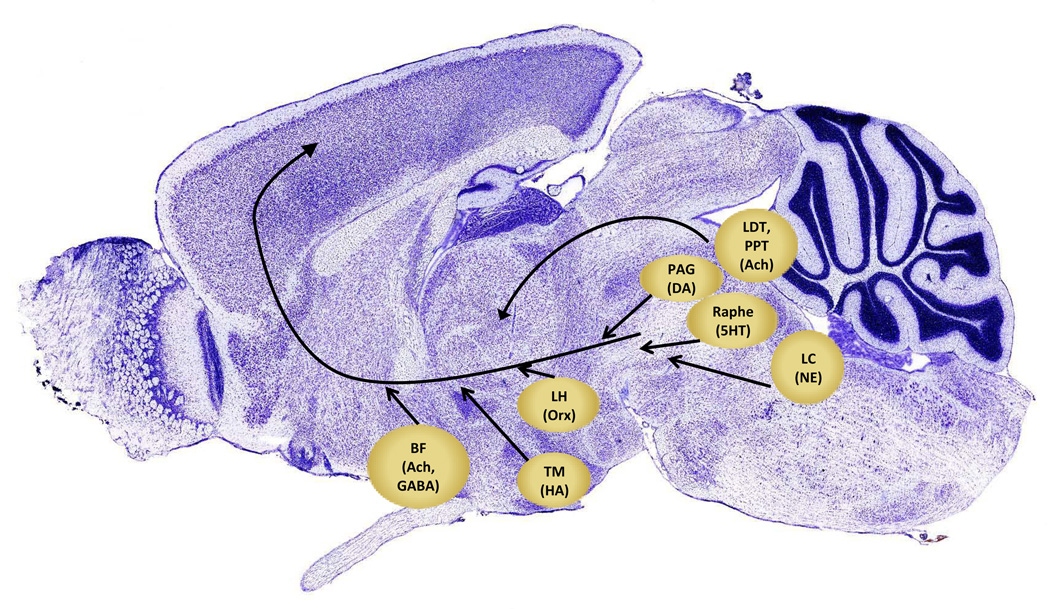
Some studies suggest that the experience of fatigue is mirrored in the brain via changes in circuits that control arousal and motivation. The ascending arousal system, one such circuit, involves many lower brain areas providing excitatory drive to thalamus and cortex. This system consists of multiple groups of neurons. Cholinergic neurons projecting to thalamus, lateral hypothalamus (LH), basal forebrain (BF) and prefrontal cortex arise from the peduculopontine tegmentum (PPT) and the laterodorsal tegmentum (LDT). Monoaminergic cell groups project to the forebrain as well, including noradrenergic locus coeruleus (LC) neurons as well as serotonergic medial and dorsal raphe neurons, and dopaminergic neurons adjacent to the dorsal raphe. Histaminergic neurons in the tuberomammillary nuclei (TMN) show similar projections and arousal-related firing patterns. These ascending projections target thalamus, LH, BF, and cerebral cortex (especially the prefrontal cortex)(Saper et al., 2010). Figure 4 outlines some of these circuits known to control arousal.
Figure 4.
Neuroanatomical areas important in the ascending arousal system. This system consists of noradrenergic neurons of the locus coeruleus (LC), cholinergic neurons of the pedunculopontine and lateraodorsal tegmental nuclei (LDT, PPT), serotonergic neurons in the raphe nuclei, dopaminergic neurons in periaqueductal gray (PAG), histaminergic neurons of the tuberomammaillary nucleus (TM), orexin-containing neurons of the lateral hypothalamus (LH) and basal forebrain (BF) neurons containing GABA and acetylcholine. (based on Fuller et al., 2006; brain image from Allen Brain Atlas).
The alternation between sleep and wake states involves switches in activity of several cell groups within this system. Wakefulness is maintained by the orexin-containing neurons in the LH; these neurons provide excitatory input to TMN neurons, serotonergic raphe nucleus neurons, and noradrenergic LC neurons, as well as to the entire cerebral cortex. These neurons receive inputs from many components of the ascending arousal system as well as from prefrontal, amygdaloid, and VTA sites (Saper et al., 2010). Orexin neurons in the lateral hypothalamus appear to be involved both in networks that maintain wake and also those that mediate reward-seeking, such as those in the VTA (Aston-Jones et al., 2010), with different neurons within the orexin-expressing group subserving these different functions. Orexin neurons project to neurons in the VTA where they modulate the effects of prefrontal cortex inputs to the dopamine-containing VTA neurons (Moorman and Aston-Jones, 2010). Sleep is controlled in part by cells in the preoptic area (POA) that can inhibit cells in the TM, raphe and LC (Rosenwasser, 2009). Cells in the ventrolateral preoptic area (VLPO) appear to serve an "executive" function in inducing sleep (Rosenwasser, 2009). Following lesions to VLPO neurons animals show less time asleep and more fragmented sleep bouts (Lu et al., 2000).
Cytokines can directly influence these circuits. Both IL-1β and TNF-α regulate sleep in the normal animal (Imeri and Opp, 2009) with levels in the brain increasing around the time of day when the animal is likely to sleep. IL-1β directly inhibits wake-promoting neurons and stimulates sleep-promoting neurons in the POA and basal forebrain (BF) (Imeri and Opp, 2009). IL-1β inhibits serotonergic neurons of the dorsal raphe nuclei by enhancing the effects of the inhibitory neurotransmitter GABA (Imeri and Opp, 2009). IL-1β also stimulates 5-HT release from axon terminals in the POA which stimulates sleep and serotonin stimulates IL-1β mRNA transcription in the hypothalamus, providing positive feedback.
Studies using animal models have identified several of these brain regions as important in effects of inflammation on neural circuits driving motor activity. For example, LPS-induced acute suppression of motor activity (circa 1.5 h following LPS administration) was linked to reduced activation in the dopaminergic VTA and histaminergic TMN and orexin neurons in the LH (Gaykema and Goehler, 2011). Decreased serotonergic drive is implicated in another model. Fatigue eight days following administration of poly I:C is accompanied by increased IFN-α, p38 mitogen-activated protein kinase (MAPK), and serotonin transporter expression in lateral POA and median preoptic area (MnPO) and as well as cortex (Katafuchi et al., 2005). Microinjection of IFN-α into the prefrontal cortex led to a decrease in serotonin levels (Katafuchi et al., 2006). Administration of the 5-HT1A agonist 8-OH-DPAT, but not 5-HT2, 5-HT3, or dopamine D3 agonists, was able to reduce the effect of poly I:C on wheel-running. These studies point to many of the structures shown in Figure 4 as being altered in the brain of an animal experiencing fatigue.
3.3 Cytokine-induced suppression of circadian pacemaker output
Suppression of neural drive from the circadian clock could underlie chronic fatigue. It would be expected that a muffled daily signal from the circadian pacemaker might lead to reduced energy levels, impaired mental concentration and increased fatigue and fatigability. This might also lead to loss of internal synchrony among brain areas normally expressing circadian rhythms in neural activity, potentially impairing function of many brain regions. Loss of synchrony among peripheral oscillators could bring about a general feeling of malaise similar to that experienced during jet lag or rotating shift work. Loss of circadian organization will impact metabolism and immune system function. Reduced locomotor activity and/or disrupted sleep associated with feelings of fatigue could reduce the feedback that locomotor activity normally provides to sculpt daily rhythmic output, further damping rhythm output.
Circadian rhythms are controlled by a pacemaker in the suprachiasmatic nuclei (SCN) in the hypothalamus. A clinical report of a case study with damage to the circadian pacemaker in the SCN suggests that such damage does leave a person feeling fatigued (Cohen and Albers, 1991). A study in squirrel monkeys indicates that SCN lesions reduce total wakefulness (Edgar et al., 1993), suggesting the SCN may function to increase wakefulness during the active period. The SCN may also function to increase sleep during the inactive period (Mistlberger, 2005). Damage to the SCN in rats abolishes scale-invariant temporal patterns in locomotor activity over scales of minutes to days (Hu et al., 2007), indicating the wide influence of this small nucleus in coordinating activity of many other neural nodes. As can be seen in Figure 5, the SCN is interconnected with regions important in regulating sleep/wake, arousal, and reward discussed above. Projections from the suprachiasmatic nuclei (SCN) reach both the wake-promoting neurons and the sleep-promoting systems by both direct and indirect routes, relayed through the dorsomedial hypothalamus (DMH) and the ventral subparaventricular zone (Rosenwasser, 2009). Lesions of the DMH, a major target of SCN efferents, reduces total time awake (Chou et al., 2003). The neurons of the SCN also project to orexin-containing neurons in the DMH and then to the LC, a structure that modulates arousal by noradrenergic innervation of many regions including the frontal cortex (Benca et al., 2009).The SCN projects to the VTA, likely via a relay in the MnPO, and a sub-population of VTA neurons shows diurnal modulation of firing rate (Luo and Aston-Jones, 2009); this circuit might mediate circadian regulation of reward-seeking.
Figure 5.
SCN connections to sleep circuits. Several select afferent inputs to the SCN are shown, the photic input via the retina and inputs from raphe nuclei and intergeniculate leaflet (IGL) that appear to have more to do with mediating influences of activity or arousal on the SCN. Many efferents from the SCN terminate in the sub-paraventricular zone (sPVZ) and then converge with direct efferents on cells in the dorsomedial hypothalamus (DMH). Both direct and indirect SCN efferents impact the VLPO, where GABAergic neurons play an important role in inducing sleep. Indirect SCN efferent pathways target the LH where orexin-containing neurons help switch to a state of wake. Cells in the DMH target the LC where noradrenergic (NE) neurons help maintain arousal. Outputs from the SCN indirectly impinge on the VTA to modify reward circuits.
The SCN is responsive to cytokines. Receptors for IFN-γ are found in the ventrolateral SCN of rats (Lundkvist et al., 1998), and in vitro studies show effects of LPS combined with TNF-α and IFN-γ on excitatory postsynaptic activity and the amplitude of the circadian rhythm in firing rate in rat SCN (Lundkvist et al., 2002). The cytokine TNF-α is able to excite a subset of SCN neurons modulating the spontaneous firing and synaptic transmission in the SCN (Nygard et al., 2009). The suppressors of cytokine signaling (SOCS) 1 and 3 are also expressed in the mouse SCN (Sadki et al., 2007). Cells in the SCN express and respond to several pro-inflammatory cytokines in an age-dependent manner (Beynon and Coogan, 2010, Cavadini et al., 2007, Kwak et al., 2008, Sadki et al., 2007).Injections of the cytokines IFN-γ and TNF-α icv in mice elicits activation of SCN microglia (Bentivoglio et al., 2006). LPS can induce a phase shift in mouse locomotor activity and the expression of c-Fos in the SCN (Marpegan et al., 2005). Reduced amplitude circadian rhythms following infection with simian immunodeficiency virus is associated with activated microglia in the subparaventricular zone, a target for many SCN efferents (Huitron-Resendiz et al., 2007).
In multiple neurological disorders where people report fatigue and animal models show reduced locomotor activity, the SCN shows reduced levels of daytime firing rate and/or reduced amplitude rhythm in spontaneous firing. For example, animal models for Parkinson's disease (mice with over-expression of alpha-synuclein) show reduced amplitude wheel-running rhythms and reduced firing rate recorded from the SCN but surprisingly little change in the clock gene Per2 (Kudo et al., 2011a). Similar results were obtained in transgenic mice expressing the entire human Huntington's Disease gene (Kudo et al., 2011b). Rats infected with the parasite Trypanosoma brucei show reduced amplitude circadian activity rhythms and the SCN spontaneous firing rate rhythm is dampened as well (Lundkvist et al., 1998, Lundkvist et al., 2010).
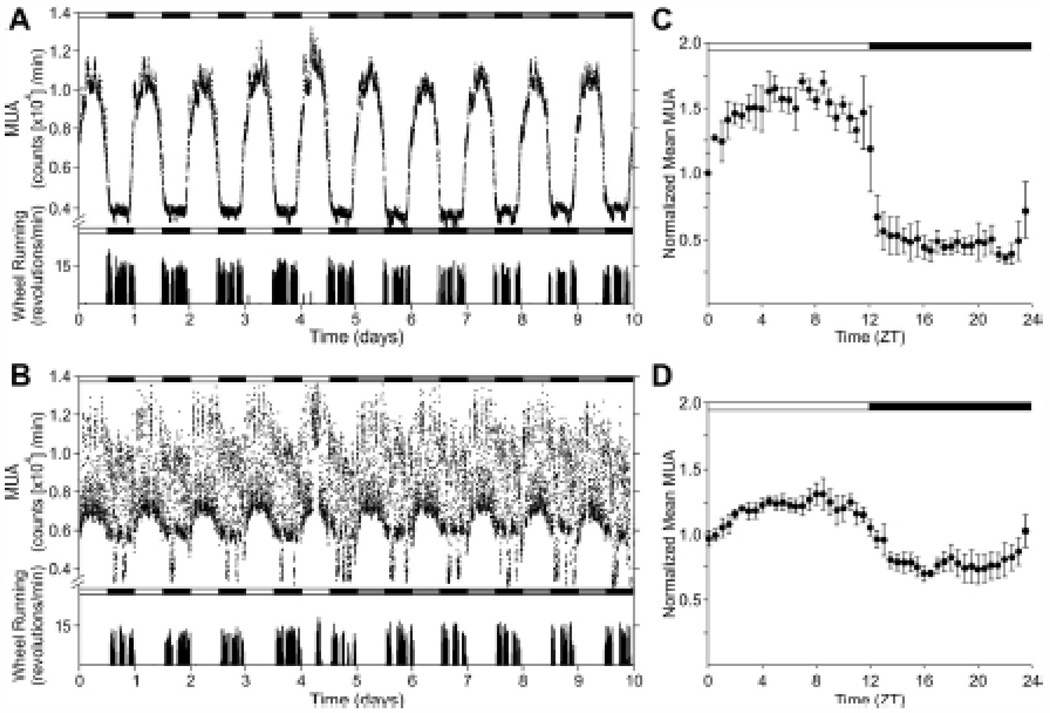
Older humans show alterations in the circadian timing system, including reduced amplitude of rhythms (Monk et al., 1995, Munch et al., 2005). Healthy older adults show disruptions of the pattern of sleep and wake which include difficulty initiating sleep, frequent night time awakenings, early morning waking and high sleep propensity during the latter half of the day (Bliwise, 1993). Aged mice show increased activated microglia and astrocytes in the SCN both in control conditions and following icv injection of cytokines IFN-γ and TNF-α (Deng et al., 2010). Aged animals show decreased amplitude rhythms of SCN electrical activity (Nakamura et al., 2011, Satinoff et al., 1993, Watanabe et al., 1995) which some researchers have proposed might be due to action of cytokines on the SCN (Coogan and Wyse, 2008; see Figure 6).
Figure 6.
Older animals show reduced amplitude SCN neural firing and reduced locomotor activity. Neural firing recorded in vivo (multiple unit activity, MUA) and locomotor activity of young (A) and middle aged (15±2 months) mice (B). Top bars indicate light (open) and dark (shaded) lighting conditions. Integrated mean MUA activity for each age group is shown in C and D. (N=4/group; from Nakamura et al., 2011).
Interestingly, studies of the genes driving circadian oscillators via transcription-translation feedback loops (Ko and Takahashi, 2006) show only relatively minor alterations due to age (Asai et al., 2001, Kolker et al., 2003, Nakamura et al., 2011), neurodegenerative disease (Kudo et al., 2011a, Kudo et al., 2011b) or T. brucei infection (Lundkvist et al., 1998, Lundkvist et al., 2010). A recent review suggested that, instead of molecular rhythms, "neural activity rhythms in the SCN may be the 'weak link' of the circadian system" (Colwell, 2011). Examples where the molecular clock seems to be functioning but neural firing output is dampened suggest that we might be well advised to develop treatments that boost cell firing from the SCN to better restore daily activity rhythms and normal levels of locomotor activity in these populations.
However, while some cases of fatigue seem to involve suppression of neural firing but not suppression of clock genes in the SCN, in other instances clock genes are clearly affected. For example, IFN-α has dramatic effects on clock genes in neurons of the SCN, suppressing expression of key clock genes Clock, Bmal1, and Period (Koyanagi and Ohdo, 2002, Ohdo et al., 2001). Injections of IFN-α or IFN-γ show a time-of-day-dependent effect on circadian activity rhythm amplitude and clock gene expression in the SCN (Ohdo et al., 2001). A different cytokine, TNF-α, suppresses expression of clock genes driven by E-box promoters in fibroblasts (Cavadini et al., 2007); this may explain effects of CD40 antibody as well as some aspects of LPS-driven immune activation (Okada et al., 2008, Taraborrelli et al., 2011).
Interestingly, some cytokines may be produced by SCN cells, and function in clock output pathways. For example, TGF-α is expressed at high levels in the SCN of Syrian hamster, rats, mice, and rhesus macaques (Kramer et al., 2001, Li et al., 2002, Ma et al., 1994, Van der Zee et al., 2005). TGF-α, infused into the third ventricle, reversibly inhibits locomotor activity, an action thought to be mediated by the EGFR (ErbB-1) on hypothalamic neurons (Kramer et al., 2001). A more long term TGF-α infusion leads to reversible inhibition of grooming, exploring and feeding and to significant weight loss (Snodgrass-Belt et al., 2005). EGFR is widely expressed in the brain and is present in the cells of the subparaventricular zone, a major target for SCN efferents, just dorsal to the SCN (Kramer et al., 2001) as well as in the SCN (Jobst et al., 2004, Van der Zee et al., 2005). Similar effects are reported for neuregulin-1 that acts via the ErbB4 receptor (Snodgrass-Belt et al., 2005) and the cardiotrophin-like cytokine, a cytokine in the IL-6 family that utilizes the gp130 receptor (Kraves and Weitz, 2006). The current model is that these cytokines act as signaling molecules that provide the daily signal for rest from the circadian pacemaker in the SCN. Microglia activation leading to increased production of cytokines in the hypothalamus may simply stimulate these circuits used by SCN neurons to drive rest.
Another important output molecule for the SCN is prokineticin 2 (PK2). This is synthesized by SCN neurons and is found in many regions targeted by SCN efferents (Zhang et al., 2009). Interestingly, deletion of either the PK2 gene or its receptor suppresses the amplitude of circadian locomotor activity rhythms (Li et al., 2006, Prosser et al., 2007). Suppression of SCN firing rate via tetrodotoxin reduces levels of PK2 in the SCN (Baba et al., 2008). Thus, reduced cell firing will lead to reduced PK2 levels, which may then augment reductions in locomotor activity. Cells in the SCN also express vasoactive intestinal polypeptide (VIP) and vasopressin in output projections. Administration of LPS to the SCN brain slice induces a dose-dependent increase in vasopressin release (Nava et al., 2000). VIP is important in SCN cell coupling as well as SCN cell output (Vosko et al., 2007). Mice deficient in this peptide show decreased locomotor activity, but daily voluntary exercise can help organize the circadian rhythm in these mice (Hannibal et al., 2011, Power et al., 2010).
4. Future directions
Future research will likely expand our understanding of the role of chemokines which are highlighted in a few recent studies. Mice treated with poly I:C show upregulation of multiple chemokines and their receptors across many brain areas, with the cerebellum particularly sensitive (Fil et al., 2011). B. abortus can cause astrocyte proliferation and apoptosis, secretion of pro-inflammatory cytokines and chemokines in the brain(Garcia Samartino et al., 2010). We need to better understand what role chemokines play in modulating these neural circuits underlying fatigue.
Stronger experiments to better establish some of the suggestions from the current literature would provide more robust foundations for our understanding. The strongest evidence for an important role of the circadian pacemaker in fatigue arises from several models (aging, neurodegenerative disorders, T. brucei infection) and an immediate concern is to see these findings expanded to immunologically based models such as poly I:C or IL-1β treatments. Tests of the necessary role of any specific brain area, as would be possible through targeted infusions of an antagonist such as IL-1ra, will help researchers separate correlations from causative changes.
Fatigue appears to be linked to CNS response to immune activation. To better treat fatigue we might consider that it might be helpful to block the signal from peripheral immune challenge that causes increased cytokines in the brain. In laboratory animals treatment with a COX inhibitor, a capsase-1 inhibitor, or an IL-1β antagonist were able to reverse behavioral signs of fatigue following LPS challenge (Harden et al., 2011, Konsman et al., 2008, Teeling et al., 2007), suggesting that this might be an encouraging direction for future research. A clinical trial testing a recombinant IL-1receptor antagonist as a potential treatment for fatigue reported encouraging results in a post-hoc analysis, but the primary endpoint was not statistically significant and the sample size was small (total n=26) (Norheim et al., 2012). Further research into treatments is necessary.
Can we treat fatigue better if we conceptualize it as arising from circadian dysregulation? Fatigue in cancer patients is correlated with disrupted diurnal rhythms, reduced light exposure during the day, and delayed phase, with some preliminary results indicating benefits from light therapy (Ancoli-Israel et al., 2012). Light can have effects both from being directly activating as well as from influencing circadian rhythms. New targets for pharmacological therapies might include targets of SCN output pathways. Researchers could work to develop ways to boost cell firing rate during the daytime in the SCN or to suppress cell firing in this nucleus during the nighttime. We need to know what the effect of habitual exercise has on SCN cell firing rhythms. A recent study showed effects of 8 weeks of voluntary wheel-running on gene expression in the hippocampus(Kohman et al., 2011). Researchers cannot yet say what genetic and environmental factors predispose people to developing fatigue, but clearly some people are more susceptible than others. Interestingly, chronic fatigue syndrome is associated with a polymorphism and increased expression of the circadian clock gene NPAS2 (Smith et al., 2011). New research could examine human fibroblasts from people with fatigue to determine if screening for circadian parameters is helpful for diagnosis or treatment.
The goal in studying a symptom is not to cure a disease; of course, fatigue is caused by diverse disease processes. A full understanding of the causes of fatigue requires consideration of the multitude of health and psychosocial factors and the relevance of subject variables such as age and gender that can contribute to this symptom. We now can research fatigue using a robust set of animal models, and we hope to find, regardless of cause, common neurobiological substrates, research that should lead to better treatments.
Highlights.
Fatigue is a common symptom of many disorders but with few effective treatments.
Fatigue can be studied with animal models.
Activation of brain microglia or increased cytokines is associated with fatigue.
The neural network includes regions for arousal, sleep, reward, and circadian rhythms.
Research on neural targets may allow development of novel treatments for fatigue.
Acknowledgments
This work was supported in part by NIH grant R21-CA125215 and R21-NR012845. I am deeply grateful to colleagues Dr. William Schwartz, Dr. Megan Hastings Hagenauer, Dr. Michael Dash, and Andrew Vosko for comments on an earlier draft. I am grateful to the Helen Riaboff Whiteley Center for providing a retreat that allowed this paper to be written.
Abbreviation list
- BF
basal forebrain
- BDNF
brain-derived neurotrophic factor
- DMH
dorsomedial hypothalamus
- IGL
intergeniculate leaflet
- LH
lateral hypothalamus
- LDT
laterodorsal tegmentum
- LPS
lipopolysaccaride
- LC
locus coeruleus
- MnPO
median preoptic area
- MAPK
mitogen-activated protein kinase
- MUA
multiple unit activity
- NE
noradrenergic
- NAc
nucleus accumbens
- NTS
nucleus of the solitary tract
- PPT
peduculopontine tegmentum
- PAG
periaqueductal gray
- poly I:C
polyinosinic: polycytidylic acid
- POA
preoptic area
- PK2
prokineticin 2
- sPVZ
sub-paraventricular zone
- SCN
suprachiasmatic nuclei
- SOCS
suppressors of cytokine signaling
- TMN
tuberomammillary nuclei
- VIP
vasoactive intestinal polypeptide
- VTA
ventral tegmental area
- VLPO
ventrolateral preoptic area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav.Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Rissling M, Neikrug A, Trofimenko V, Natarajan L, Parker BA, Lawton S, Desan P, Liu L. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer. 2012;20:1211–1219. doi: 10.1007/s00520-011-1203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Gibb J, Hayley S. Influence of continuous infusion of interleukin-1beta on depression-related processes in mice: corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression. Psychopharmacology (Berl) 2008;199:231–244. doi: 10.1007/s00213-008-1166-z. [DOI] [PubMed] [Google Scholar]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J.Neurosci.Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in rewardseeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avlund K, Pedersen AN, Schroll M. Functional decline from age 80 to 85: influence of preceding changes in tiredness in daily activities. Psychosom.Med. 2003a;65:771–777. doi: 10.1097/01.psy.0000082640.61645.bf. [DOI] [PubMed] [Google Scholar]
- Avlund K, Vass M, Hendriksen C. Onset of mobility disability among community-dwelling old men and women. The role of tiredness in daily activities. Age Ageing. 2003b;32:579–584. doi: 10.1093/ageing/afg101. [DOI] [PubMed] [Google Scholar]
- Baba K, Ono D, Honma S, Honma K. A TTX-sensitive local circuit is involved in the expression of PK2 and BDNF circadian rhythms in the mouse suprachiasmatic nucleus. Eur.J.Neurosci. 2008;27:909–916. doi: 10.1111/j.1460-9568.2008.06053.x. [DOI] [PubMed] [Google Scholar]
- Bake S, Friedman JA, Sohrabji F. Reproductive age-related changes in the blood brain barrier: expression of IgG and tight junction proteins. Microvasc.Res. 2009;78:413–424. doi: 10.1016/j.mvr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17beta-Estradiol Differentially Regulates Blood-Brain Barrier Permeability in Young and Aging Female Rats. Endocrinology. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009;12:403–410. doi: 10.1089/rej.2009.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsevick AM, Cleeland CS, Manning DC, O'Mara AM, Reeve BB, Scott JA, Sloan JA ASCPRO (Assessing Symptoms of Cancer Using Patient-Reported Outcomes) ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. J.Pain Symptom Manage. 2010;39:1086–1099. doi: 10.1016/j.jpainsymman.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautmans I, Njemini R, De Backer J, De Waele E, Mets T. Surgery-induced inflammation in relation to age, muscle endurance, and self-perceived fatigue. J.Gerontol.A Biol.Sci.Med.Sci. 2010;65:266–273. doi: 10.1093/gerona/glp145. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca R, Duncan MJ, Frank E, McClung C, Nelson RJ, Vicentic A. Biological rhythms, higher brain function, and behavior: Gaps, opportunities, and challenges. Brain Res.Rev. 2009;62:57–70. doi: 10.1016/j.brainresrev.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentivoglio M, Deng XH, Nygård M, Sadki A, Kristensson K. The aging suprachiasmatic nucleus and cytokines: functional, molecular, and cellular changes in rodents. Chronobiol Int. 2006;23:437–449. doi: 10.1080/07420520500545797. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Mariotti R, Bertini G. Neuroinflammation and brain infections: historical context and current perspectives. Brain Res.Rev. 2011;66:152–173. doi: 10.1016/j.brainresrev.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Beynon AL, Coogan AN. Diurnal age, immune regulation of interleukin-1beta and interleukin-1 type 1 receptor in the mouse suprachiasmatic nucleus. Chronobiol.Int. 2010;27:1546–1563. doi: 10.3109/07420528.2010.501927. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin.Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronikowski AM, Carter PA, Swallow JG, Girard IA, Rhodes JS, Garland T., Jr Open-field behavior of house mice selectively bred for high voluntary wheel-running. Behav.Genet. 2001;31:309–316. doi: 10.1023/a:1012283426530. [DOI] [PubMed] [Google Scholar]
- Calabrese M, Rinaldi F, Grossi P, Mattisi I, Bernardi V, Favaretto A, Perini P, Gallo P. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsingremitting multiple sclerosis. Mult.Scler. 2010;16:1220–1228. doi: 10.1177/1352458510376405. [DOI] [PubMed] [Google Scholar]
- Cantor F. Central and peripheral fatigue: exemplified by multiple sclerosis and myasthenia gravis. PM R. 2010;2:399–405. doi: 10.1016/j.pmrj.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Cantor JB, Ashman T, Gordon W, Ginsberg A, Engmann C, Egan M, Spielman L, Dijkers M, Flanagan S. Fatigue after traumatic brain injury and its impact on participation and quality of life. J.Head Trauma Rehabil. 2008;23:41–51. doi: 10.1097/01.HTR.0000308720.70288.af. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol.Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNFalpha suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc.Natl.Acad.Sci.U.S.A. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, DeLaHunt M, Hu S, Close K, Peterson PK. Immunologically mediated fatigue: a murine model. Clin.Immunol.Immunopathol. 1992;64:161–165. doi: 10.1016/0090-1229(92)90194-s. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. Fatigue and basal ganglia. J.Neurol.Sci. 2000;179:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Moriya J, Yamakawa J, Takahashi T, Li Q, Morimoto S, Iwai K, Sumino H, Yamaguchi N, Kanda T. Brain atrophy in a murine model of chronic fatigue syndrome and beneficial effect of Hochu-ekki-to (TJ-41) Neurochem.Res. 2008;33:1759–1767. doi: 10.1007/s11064-008-9620-1. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J.Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christley Y, Duffy T, Martin CR. A review of the definitional criteria for chronic fatigue syndrome. J Eval Clin Pract. 2012;18:25–31. doi: 10.1111/j.1365-2753.2010.01512.x. [DOI] [PubMed] [Google Scholar]
- Clark MR, Katon W, Russo J, Kith P, Sintay M, Buchwald D. Chronic fatigue: risk factors for symptom persistence in a 2 1/2-year follow-up study. Am.J.Med. 1995;98:187–195. doi: 10.1016/S0002-9343(99)80403-3. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Albers HE. Disruption of human circadian and cognitive regulation following a discrete hypothalamic lesion: a case study. Neurology. 1991;41:726–729. doi: 10.1212/wnl.41.5.726. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early findings. Brain Behav.Immun. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat.Rev.Neurosci. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan AN, Wyse CA. Neuroimmunology of the circadian clock. Brain Res. 2008;1232:104–112. doi: 10.1016/j.brainres.2008.07.087. [DOI] [PubMed] [Google Scholar]
- Cope H. Fatigue: a non-specific complaint? International Review of Psychiatry. 1992;4:273–280. [Google Scholar]
- Corona AW, Fenn AM, Godbout JP. Cognitive and Behavioral Consequences of Impaired Immunoregulation in Aging. J Neuroimmune Pharmacol. 2012;7:7–23. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav.Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol.Allergy Clin.North.Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat.Rev.Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, Siracusano A, Castelli M, Cavasinni F, Bernardi G, Usiello A, Centonze D. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology. 2010;35:374–387. doi: 10.1038/npp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behav.Brain Res. 2009;200:128–133. doi: 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- DeFries JC, Wilson JR, McClearn GE. Open-field behavior in mice: selection response and situational generality. Behav.Genet. 1970;1:195–211. doi: 10.1007/BF01074652. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Genova HM, Capili EJ, Wylie GR. Functional neuroimaging of fatigue. Phys.Med.Rehabil.Clin.N.Am. 2009;20:325–337. doi: 10.1016/j.pmr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Deng XH, Bertini G, Palomba M, Xu YZ, Bonaconsa M, Nygard M, Bentivoglio M. Glial transcripts and immune-challenged glia in the suprachiasmatic nucleus of young and aged mice. Chronobiol.Int. 2010;27:742–767. doi: 10.3109/07420521003681498. [DOI] [PubMed] [Google Scholar]
- Droogleever Fortuyn HA, Fronczek R, Smitshoek M, Overeem S, Lappenschaar M, Kalkman J, Renier W, Buitelaar J, Lammers GJ, Bleijenberg G. Severe fatigue in narcolepsy with cataplexy. J Sleep Res. 2012;21:163–169. doi: 10.1111/j.1365-2869.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp.Neurol. 2010;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J.Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers RG, Rietberg MB, van Wegen EE, Verhoef J, Kramer SF, Terwee CB, Kwakkel G. Self-report fatigue questionnaires in multiple sclerosis, Parkinson's disease and stroke: a systematic review of measurement properties. Qual Life Res. 2011 doi: 10.1007/s11136-011-0009-2. PMID: 22012025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fil D, Borysiewicz E, Konat GW. A broad upregulation of cerebral chemokine genes by peripherally-generated inflammatory mediators. Metab.Brain Dis. 2011;26:49–59. doi: 10.1007/s11011-010-9231-9. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J.Gerontol.A Biol.Sci.Med.Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J.Biol.Rhythms. 2006;21:482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- Garcia Samartino C, Delpino MV, Pott Godoy C, Di Genaro MS, Pasquevich KA, Zwerdling A, Barrionuevo P, Mathieu P, Cassataro J, Pitossi F, Giambartolomei GH. Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. Am.J.Pathol. 2010;176:1323–1338. doi: 10.2353/ajpath.2010.090503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE. Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: brain substrates for fatigue? Brain Behav.Immun. 2011;25:443–460. doi: 10.1016/j.bbi.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber LH. Some unresolved issues for the study of fatigue: the way forward. PM R. 2010;2:466–468. doi: 10.1016/j.pmrj.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O'Connor J, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl EJ, Huang MC, Kon J, Patel K, Schwartz JB, Fast K, Ferrucci L, Madara K, Taub DD, Longo DL. Gender specificity of altered human immune cytokine profiles in aging. FASEB J. 2010;24:3580–3589. doi: 10.1096/fj.10-160911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Hsiung HM, Fahrenkrug J. Temporal phasing of locomotor activity, heart rate rhythmicity, and core body temperature is disrupted in VIP receptor 2-deficient mice. Am.J.Physiol.Regul.Integr.Comp.Physiol. 2011;300:R519–R530. doi: 10.1152/ajpregu.00599.2010. [DOI] [PubMed] [Google Scholar]
- Harden LM, du Plessis I, Roth J, Loram LC, Poole S, Laburn HP. Differences in the relative involvement of peripherally released interleukin (IL)-6, brain IL-1beta and prostanoids in mediating lipopolysaccharide-induced fever and sickness behavior. Psychoneuroendocrinology. 2011;36:608–622. doi: 10.1016/j.psyneuen.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hardy SE, Studenski SA. Fatigue predicts mortality in older adults. J.Am.Geriatr.Soc. 2008;56:1910–1914. doi: 10.1111/j.1532-5415.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health.Qual.Life.Outcomes. 2007;5:12. doi: 10.1186/1477-7525-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneber M, Fischer I, Dimeo F, Rüffer JU, Weis J. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109:161–71. doi: 10.3238/arztebl.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Scheer FA, Ivanov PC, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149:508–517. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitron-Resendiz S, Marcondes MC, Flynn CT, Lanigan CM, Fox HS. Effects of simian immunodeficiency virus on the circadian rhythms of body temperature and gross locomotor activity. Proc Natl Acad Sci U S A. 2007;104:15138–15143. doi: 10.1073/pnas.0707171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L, Ceccarelli P, Mariotti M, Manfridi A, Opp MR, Mancia M. Sleep, but not febrile responses of Fisher 344 rats to immune challenge are affected by aging. Brain Behav Immun. 2004;18:399–404. doi: 10.1016/j.bbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat.Rev.Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Pierre P, Morrow GR, Roscoe JA, Heckler C, Mohile S, Janelsins M, Peppone L, Hemstad A, Esparaz BT, Hopkins JO. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116:3513–3520. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst EE, Robinson DW, Allen CN. Potential pathways for intercellular communication within the calbindin subnucleus of the hamster suprachiasmatic nucleus. Neuroscience. 2004;123:87–99. doi: 10.1016/j.neuroscience.2003.08.059. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Kondo T, Take S, Yoshimura M. Brain cytokines and the 5-HT system during poly I:C-induced fatigue. Ann.N.Y.Acad.Sci. 2006;1088:230–237. doi: 10.1196/annals.1366.020. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Kondo T, Take S, Yoshimura M. Enhanced expression of brain interferon-alpha and serotonin transporter in immunologically induced fatigue in rats. Eur.J.Neurosci. 2005;22:2817–2826. doi: 10.1111/j.1460-9568.2005.04478.x. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Kondo T, Yasaka T, Kubo K, Take S, Yoshimura M. Prolonged effects of polyriboinosinic:polyribocytidylic acid on spontaneous running wheel activity and brain interferon-alpha mRNA in rats: a model for immunologically induced fatigue. Neuroscience. 2003;120:837–845. doi: 10.1016/s0306-4522(03)00365-8. [DOI] [PubMed] [Google Scholar]
- Kirsh KL, Passik S, Holtsclaw E, Donaghy K, Theobald D. I get tired for no reason: a single item screening for cancer-related fatigue. J.Pain Symptom Manage. 2001;22:931–937. doi: 10.1016/s0885-3924(01)00350-5. [DOI] [PubMed] [Google Scholar]
- Knab AM, Lightfoot JT. Does the difference between physically active and couch potato lie in the dopamine system? Int.J.Biol.Sci. 2010;6:133–150. doi: 10.7150/ijbs.6.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop H, van Kessel K, Moss-Morris R. Which cognitions and behaviours mediate the positive effect of cognitive behavioural therapy on fatigue in patients with multiple sclerosis? Psychol Med. 2012;42:205–13. doi: 10.1017/S0033291711000924. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum.Mol.Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Rodriguez-Zas SL, Southey BR, Kelley KW, Dantzer R, Rhodes JS. Voluntary wheel running reverses age-induced changes in hippocampal gene expression. PLoS One. 2011;6:e22654. doi: 10.1371/journal.pone.0022654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J.Biol.Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kohsaka S, Okabe S. Long-term changes of spine dynamics and microglia after transient peripheral immune response triggered by LPS in vivo. Mol.Brain. 2011;4:27. doi: 10.1186/1756-6606-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Veeneman J, Combe C, Poole S, Luheshi GN, Dantzer R. Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur.J.Neurosci. 2008;28:2499–2510. doi: 10.1111/j.1460-9568.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- Kos D, Kerckhofs E, Nagels G, D'hooghe MB, Ilsbroukx S. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil.Neural Repair. 2008;22:91–100. doi: 10.1177/1545968306298934. [DOI] [PubMed] [Google Scholar]
- Koyanagi S, Ohdo S. Alteration of intrinsic biological rhythms during interferon treatment and its possible mechanism. Mol.Pharmacol. 2002;62:1393–1399. doi: 10.1124/mol.62.6.1393. [DOI] [PubMed] [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- Kraves S, Weitz CJ. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat.Neurosci. 2006;9:212–219. doi: 10.1038/nn1633. [DOI] [PubMed] [Google Scholar]
- Kristensson K, Nygard M, Bertini G, Bentivoglio M. African trypanosome infections of the nervous system: parasite entry and effects on sleep and synaptic functions. Prog.Neurobiol. 2010;91:152–171. doi: 10.1016/j.pneurobio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson's disease. Exp.Neurol. 2011a;232:66–75. doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Kudo T, Schroeder A, Loh DH, Kuljis D, Jordan MC, Roos KP, Colwell CS. Dysfunctions in circadian behavior and physiology in mouse models of Huntington's disease. Exp.Neurol. 2011b;228:80–90. doi: 10.1016/j.expneurol.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Lundkvist GB, Brask J, Davidson A, Menaker M, Kristensson K, Block GD. Interferon-gamma alters electrical activity and clock gene expression in suprachiasmatic nucleus neurons. J.Biol.Rhythms. 2008;23:150–159. doi: 10.1177/0748730407313355. [DOI] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J.Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sankrithi N, Davis FC. Transforming growth factor-alpha is expressed in astrocytes of the suprachiasmatic nucleus in hamster: role of glial cells in circadian clocks. Neuroreport. 2002;13:2143–2147. doi: 10.1097/00001756-200211150-00031. [DOI] [PubMed] [Google Scholar]
- Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.02.001. Mar 2. [Epub ahead of print] PubMed PMID: 22406004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Rissling M, Natarajan L, Fiorentino L, Mills PJ, Dimsdale JE, Sadler GR, Parker BA, Ancoli-Israel S. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep. 2012;35:237–245. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J.Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Christenson J, ElTayeb RA, Peng ZC, Grillner P, Mhlanga J, Bentivoglio M, Kristensson K. Altered neuronal activity rhythm and glutamate receptor expression in the suprachiasmatic nuclei of Trypanosoma brucei-infected rats. J.Neuropathol.Exp.Neurol. 1998;57:21–29. doi: 10.1097/00005072-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Hill RH, Kristensson K. Disruption of circadian rhythms in synaptic activity of the suprachiasmatic nuclei by African trypanosomes and cytokines. Neurobiol.Dis. 2002;11:20–27. doi: 10.1006/nbdi.2002.0536. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Robertson B, Mhlanga JD, Rottenberg ME, Kristensson K. Expression of an oscillating interferon-gamma receptor in the suprachiasmatic nuclei. Neuroreport. 1998;9:1059–1063. doi: 10.1097/00001756-199804200-00018. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Sellix MT, Nygard M, Davis E, Straume M, Kristensson K, Block GD. Clock gene expression during chronic inflammation induced by infection with Trypanosoma brucei brucei in rats. J.Biol.Rhythms. 2010;25:92–102. doi: 10.1177/0748730409360963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Aston-Jones G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: a novel circadian output pathway. Eur.J.Neurosci. 2009;29:748–760. doi: 10.1111/j.1460-9568.2008.06606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YJ, Berg-von der Emde K, Moholt-Siebert M, Hill DF, Ojeda SR. Regionspecific regulation of transforming growth factor alpha (TGF alpha) gene expression in astrocytes of the neuroendocrine brain. J.Neurosci. 1994;14:5644–5651. doi: 10.1523/JNEUROSCI.14-09-05644.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegán L, Bekinschtein TA, Costas MA, Golombek DA. Circadian responses to endotoxin treatment in mice. J Neuroimmunol. 2005;160:102–109. doi: 10.1016/j.jneuroim.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland T, Jr, Pomp D. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav.Brain Res. 2010;210:155–163. doi: 10.1016/j.bbr.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res.Brain Res.Rev. 2005;49:429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, 3rd, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Exp.Gerontol. 1995;30:455–474. doi: 10.1016/0531-5565(95)00007-4. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J.Gerontol.A Biol.Sci.Med.Sci. 2010;65:887–895. doi: 10.1093/gerona/glq064. [DOI] [PubMed] [Google Scholar]
- Moriya J, Chen R, Yamakawa J, Sasaki K, Ishigaki Y, Takahashi T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol.Pharm.Bull. 2011;34:354–359. doi: 10.1248/bpb.34.354. [DOI] [PubMed] [Google Scholar]
- Morley JE, Baumgartner RN. Cytokine-related aging process. J.Gerontol.A Biol.Sci.Med.Sci. 2004;59:M924–M929. doi: 10.1093/gerona/59.9.m924. [DOI] [PubMed] [Google Scholar]
- Morrow GR, Shelke AR, Roscoe JA, Hickok JT, Mustian K. Management of cancer-related fatigue. Cancer Invest. 2005;23:229–239. doi: 10.1081/cnv-200055960. [DOI] [PubMed] [Google Scholar]
- Munch M, Knoblauch V, Blatter K, Schroder C, Schnitzler C, Krauchi K, Wirz-Justice A, Cajochen C. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol.Aging. 2005;26:1307–1319. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-related decline in circadian output. J.Neurosci. 2011;31:10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava F, Carta G, Haynes LW. Lipopolysaccharide increases arginine-vasopressin release from rat suprachiasmatic nucleus slice cultures. Neurosci.Lett. 2000;288:228–230. doi: 10.1016/s0304-3940(00)01199-x. [DOI] [PubMed] [Google Scholar]
- Nijhof SL, Bleijenberg G, Uiterwaal CS, Kimpen JL, van de Putte EM. Effectiveness of internet-based cognitive behavioural treatment for adolescentswith chronic fatigue syndrome (FITNET): a randomised controlled trial. Lancet. 2012;379:1412–1418. doi: 10.1016/S0140-6736(12)60025-7. [DOI] [PubMed] [Google Scholar]
- Norheim KB, Harboe E, Gøransson LG, Omdal R. Interleukin-1 inhibition andfatigue in primary Sjögren's syndrome--a double blind, randomised clinical trial. PLoS One. 2012;7:e30123. doi: 10.1371/journal.pone.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard M, Lundkvist GB, Hill RH, Kristensson K. Rapid nitric oxide-dependent effects of tumor necrosis factor-alpha on suprachiasmatic nuclei neuronal activity. Neuroreport. 2009;20:213–217. doi: 10.1097/WNR.0b013e32831f1ca2. [DOI] [PubMed] [Google Scholar]
- Ohdo S, Koyanagi S, Suyama H, Higuchi S, Aramaki H. Changing the dosing schedule minimizes the disruptive effects of interferon on clock function. Nat.Med. 2001;7:356–360. doi: 10.1038/85507. [DOI] [PubMed] [Google Scholar]
- Okada K, Yano M, Doki Y, Azama T, Iwanaga H, Miki H, Nakayama M, Miyata H, Takiguchi S, Fujiwara Y, Yasuda T, Ishida N, Monden M. Injection of LPS causes transient suppression of biological clock genes in rats. J.Surg.Res. 2008;145:5–12. doi: 10.1016/j.jss.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Olivadoti MD, Weinberg JB, Toth LA, Opp MR. Sleep and fatigue in mice infected with murine gammaherpesvirus 68. Brain Behav.Immun. 2011;25:696–705. doi: 10.1016/j.bbi.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenweller JE, Natelson BH, Gause WC, Carroll KK, Beldowicz D, Zhou XD, LaManca JJ. Mouse running activity is lowered by Brucella abortus treatment: a potential model to study chronic fatigue. Physiol.Behav. 1998;63:795–801. doi: 10.1016/s0031-9384(97)00539-8. [DOI] [PubMed] [Google Scholar]
- Patro IK, Amit Shrivastava M, Bhumika S, Patro N. Poly I:C induced microglial activation impairs motor activity in adult rats. Indian.J.Exp.Biol. 2010;48:104–109. [PubMed] [Google Scholar]
- Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. Fatigue in Parkinson's disease is linked to striatal and limbic serotonergic dysfunction. Brain. 2010;133:3434–3443. doi: 10.1093/brain/awq268. [DOI] [PubMed] [Google Scholar]
- Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120:277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- Power A, Hughes AT, Samuels RE, Piggins HD. Rhythm-promoting actions of exercise in mice with deficient neuropeptide signaling. J.Biol.Rhythms. 2010;25:235–246. doi: 10.1177/0748730410374446. [DOI] [PubMed] [Google Scholar]
- Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database Syst.Rev. 2008;(3):CD001027. doi: 10.1002/14651858.CD001027.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Chesham JE, Ebling FJ, Hastings MH, Maywood ES. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc.Natl.Acad.Sci.U.S.A. 2007;104:648–653. doi: 10.1073/pnas.0606884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MH, Vetrivelan R, Fuller PM, Lu J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur.J.Neurosci. 2010;31:499–507. doi: 10.1111/j.1460-9568.2009.07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG, McElhiney MC, Rabkin R. Modafinil and armodafinil treatment for fatigue for HIV-positive patients with and without chronic hepatitis. CInt.J.STD AIDS. 2011;22:95–101. doi: 10.1258/ijsa.2010.010326. [DOI] [PubMed] [Google Scholar]
- Ray M, Rogers LQ, Trammell RA, Toth LA. Fatigue and sleep during cancer and chemotherapy: translational rodent models. Comp.Med. 2008;58:234–245. [PMC free article] [PubMed] [Google Scholar]
- Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA. Development of a mouse model for assessing fatigue during chemotherapy. Comp.Med. 2011;61:119–130. [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. Integr.Comp.Biol. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav.Neurosci. 2003;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Roberts MD, Gilpin L, Parker KE, Childs TE, Will MJ, Booth FW. Dopamine D1 receptor modulation in nucleus accumbens lowers voluntary wheel running in rats bred to run high distances. Physiol.Behav. 2011;105:661–668. doi: 10.1016/j.physbeh.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM. Functional neuroanatomy of sleep and circadian rhythms. Brain Res.Rev. 2009;61:281–306. doi: 10.1016/j.brainresrev.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Sadki A, Bentivoglio M, Kristensson K, Nygard M. Suppressors, receptors and effects of cytokines on the aging mouse biological clock. Neurobiol.Aging. 2007;28:296–305. doi: 10.1016/j.neurobiolaging.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, Winters A, Juan T, Li H, Begley CG, Molineux G. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- Satinoff E, Li H, Tcheng TK, Liu C, McArthur AJ, Medanic M, Gillette MU. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am.J.Physiol. 1993;265:R1216–R1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav.Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim.Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J.Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets EM, Garssen B, Bonke B, de Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- Smith AK, Fang H, Whistler T, Unger ER, Rajeevan MS. Convergent genomic studies identify association of GRIK2 and NPAS2 with chronic fatigue syndrome. Neuropsychobiology. 2011;64:183–194. doi: 10.1159/000326692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass-Belt P, Gilbert JL, Davis FC. Central administration of transforming growth factor-alpha and neuregulin-1 suppress active behaviors and cause weight loss in hamsters. Brain Res. 2005;1038:171–182. doi: 10.1016/j.brainres.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Nakamura F, Mizokawa S, Matsumura A, Nozaki S, Watanabe Y. Establishment and assessment of a rat model of fatigue. Neurosci.Lett. 2003;352:159–162. doi: 10.1016/j.neulet.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Tang WK, Chen YK, Mok V, Chu W, Ungvari GS, Ahuja AT, Wong KS. Acute basal ganglia infarcts in poststroke fatigue: an MRI study. J Neurol. 2010;257:178–82. doi: 10.1007/s00415-009-5284-2. [DOI] [PubMed] [Google Scholar]
- Taraborrelli C, Palchykova S, Tobler I, Gast H, Birchler T, Fontana A. TNFR1 is essential for CD40, but not for lipopolysaccharide-induced sickness behavior and clock gene dysregulation. Brain Behav.Immun. 2011;25:434–442. doi: 10.1016/j.bbi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Tarr AJ, McLinden KA, Kranjac D, Kohman RA, Amaral W, Boehm GW. The effects of age on lipopolysaccharide-induced cognitive deficits and interleukin-1beta expression. Behav.Brain Res. 2011;217:481–485. doi: 10.1016/j.bbr.2010.10.036. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav.Immun. 2007;21:836–850. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Teske JA, Billington CJ, Kotz CM. Hypocretin/orexin and energy expenditure. Acta Physiol.(Oxf) 2010;198:303–312. doi: 10.1111/j.1748-1716.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology. 2008;87:71–90. doi: 10.1159/000110802. [DOI] [PubMed] [Google Scholar]
- Torres-Harding S, Jason LA. What is fatigue? History and epidemiology. In: DeLuca JM, editor. Fatigue as a window to the brain. Cambridge: MIT Press; 2005. pp. 3–17. [Google Scholar]
- Van der Zee EA, Roman V, Ten B.rinke O, Meerlo P. TGFalpha and AVP in the mouse suprachiasmatic nucleus: anatomical relationship and daily profiles. Brain Res. 2005;1054:159–166. doi: 10.1016/j.brainres.2005.06.075. [DOI] [PubMed] [Google Scholar]
- van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70:205–213. doi: 10.1097/PSY.0b013e3181643065. [DOI] [PubMed] [Google Scholar]
- Vestergaard S, Nayfield SG, Patel KV, Eldadah B, Cesari M, Ferrucci L, Ceresini G, Guralnik JM. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J.Gerontol.A Biol.Sci.Med.Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen.Comp.Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Wang XS. Cancer-related fatigue: clinical science. In: Cleeland CS, Fisch MJ, Dunn AJ, editors. Cancer Symptom Science: Measurement, Mechanisms, and Management. Cambridge: Cambridge University Press; 2011. pp. 110–123. [Google Scholar]
- Wang DV, Tsien JZ. Conjunctive processing of locomotor signals by the ventral tegmental area neuronal population. PLoS One. 2011;6:e16528. doi: 10.1371/journal.pone.0016528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Shibata S, Watanabe S. Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res. 1995;695:237–239. doi: 10.1016/0006-8993(95)00713-z. [DOI] [PubMed] [Google Scholar]
- Zedlitz AM, Rietveld TC, Geurts AC, Fasotti L. Cognitive and graded activity training can alleviate persistent fatigue after stroke: a randomized, controlled trial. Stroke. 2012;43:1046–1051. doi: 10.1161/STROKEAHA.111.632117. [DOI] [PubMed] [Google Scholar]
- Zhang C, Truong KK, Zhou QY. Efferent projections of prokineticin 2 expressing neurons in the mouse suprachiasmatic nucleus. PLoS One. 2009;4:e7151. doi: 10.1371/journal.pone.0007151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Yuan J, Lv S, Tu J. Effects of exercise on behavior and peripheral blood lymphocyte apoptosis in a rat model of chronic fatigue syndrome. J.Huazhong Univ.Sci.Technolog Med.Sci. 2010;30:258–264. doi: 10.1007/s11596-010-0225-y. [DOI] [PubMed] [Google Scholar]