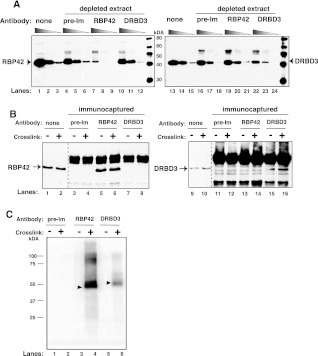
FIGURE 5.
RBP42 antibody recognizes RBP42 in cell extracts. (A) Cell extracts were depleted using either preimmune serum or RBP42 or DRBD3 antibodies. Immunoblots were probed with the indicated antibody (anti-RBP42 was used in lanes 1–12, and anti-DRBD3 was used in lanes 13–24). None indicates no-antibody-treated samples. (Lanes 1,4,7,10,13,16,19,22) Undiluted samples; (lanes 2,5,8,11,14,17,20,23) 1:5 diluted samples; and (lanes 3,6,9,12,15,18,21,24) 1:25 diluted samples from either starting or immunodepleted extracts. (B) Proteins were captured from crosslinked (+) or not crosslinked (−) parasites using the antibodies indicated above the lanes. Immunocaptured proteins were detected with anti-RBP42 (lanes 1–8) or anti-DRBD3 (lanes 9–16) antibodies. None indicates no-antibody-treated samples. (C) Immunoprecipitated protein–RNA complexes were 5′-end radiolabeled on their RNA, separated by denaturating-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. A PhosphorImager scan of protein– 32P-radiolabeled RNA complexes is shown. RBP42 and DRBD3 migrate similarly as the two proteins are similar in molecular mass, and the RNA fragments associated with the proteins are 30–50 nt long.