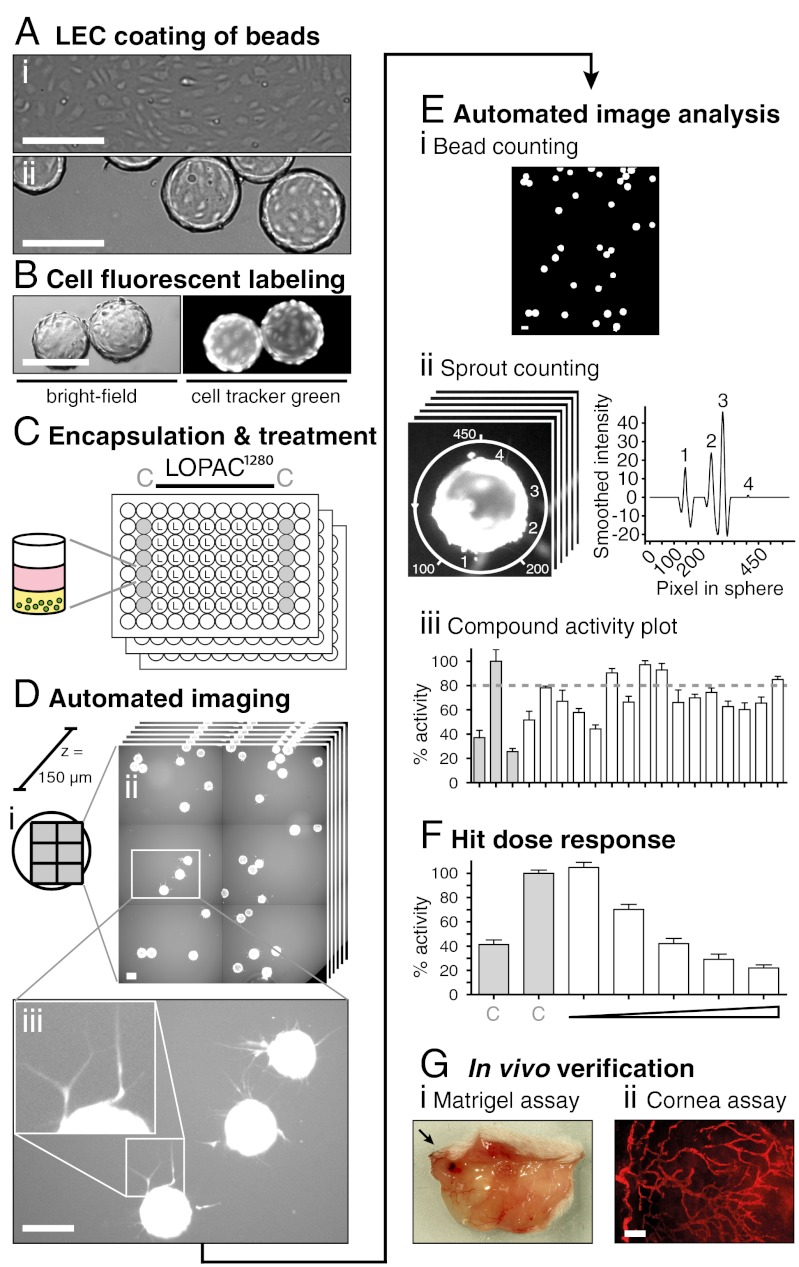
Fig. 1.
Strategy for the identification and profiling of inhibitors of lymphangiogenic sprouting in a high-content-screen. (A) Phase-contrast micrographs show cultured LECs as confluent 2D monolayers (i) and after coating onto beads as sprouting sources (ii). (B) Beads (green circles) were fluorescently labeled with cell tracker green (CTG), (C) encapsulated into collagen hydrogels (yellow), and cultured with medium (pink) supplemented with control (C) or LOPAC test compounds at 5 μM in a 96-well format. (D) Automated fluorescent scanning of plates resulted in 2 × 3 tile scans representing 80% of each well surface (i). Scans were acquired in six layers spanning the whole bead depth (150 μm) with an increment of 30 μm (ii). 24 h after treatment, LEC-coated beads showed cellular protrusions that sprouted into the collagen gel (iii). (E) Sprout number per bead was calculated by our in-house developed software SproutCounter. Beads were detected by thresholding and subsequent cluster analysis (i) and sprouts were counted by applying a sphere around the selected beads and measuring pixel intensities (Materials and Methods, Figs. S1–S3) (ii). Results are presented as “% activity compared to negative control” with an 80% cutoff defining inhibitors (iii). (F) Selected hits were reordered, retested under identical conditions, and profiled by dose response measurements, (G) before testing in two in vivo mouse models. Scale bars represent 200 μm.