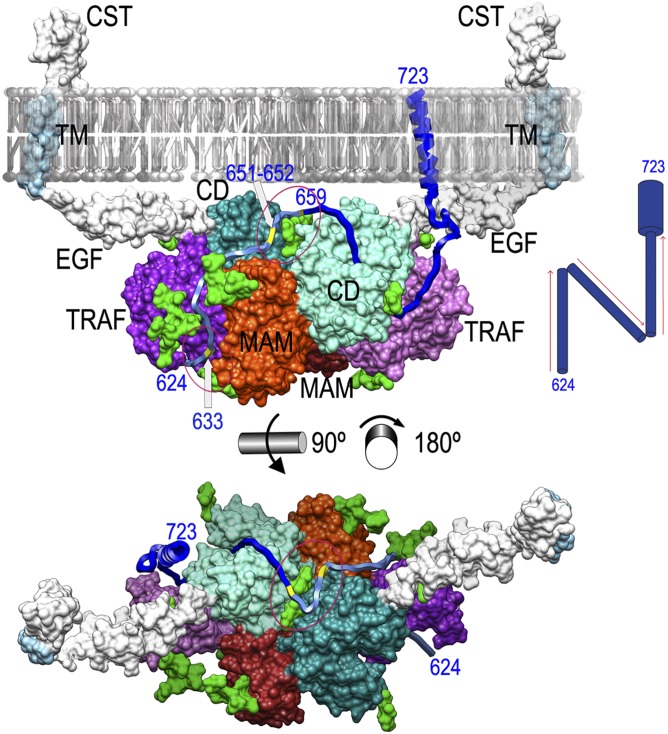
Fig. 2.
Sheddase mechanism of meprin β. Shown is a working model of Mβ function based on the experimental dimer (CDs in aquamarine and turquoise, MAMs in orange and red, TRAFs in mauve and purple, and glycosylation moieties in light green) in front (Upper) view (Fig. 1D, Left) and top (from the membrane surface; Lower) view from the membrane (here the membrane was removed for clarity). The segments present in the Mβ dimer but missing in the experimental MβΔC structure [EGF, transmembrane (TM), and cytosolic tail (CST)] have been computationally modeled (SI Materials and Methods) and are shown in white/light blue. A transmembrane substrate model for APP (segment 624–723) is shown as a blue ribbon. The substrate segment proposed to interact with the dimer partner is depicted in pale blue to highlight that this part of the mechanism is more speculative. APP glycosylation sites relevant for the proposed mechanism are marked in yellow on the ribbon and labeled. (Upper) Red ellipses highlight sugars attached to N436 of the right monomer (lower ellipse) and to N254 of both monomers (upper ellipse). (Lower) Image shows only the latter ellipse. (Upper Right Inset) A possible “N-like” trajectory of the substrate.