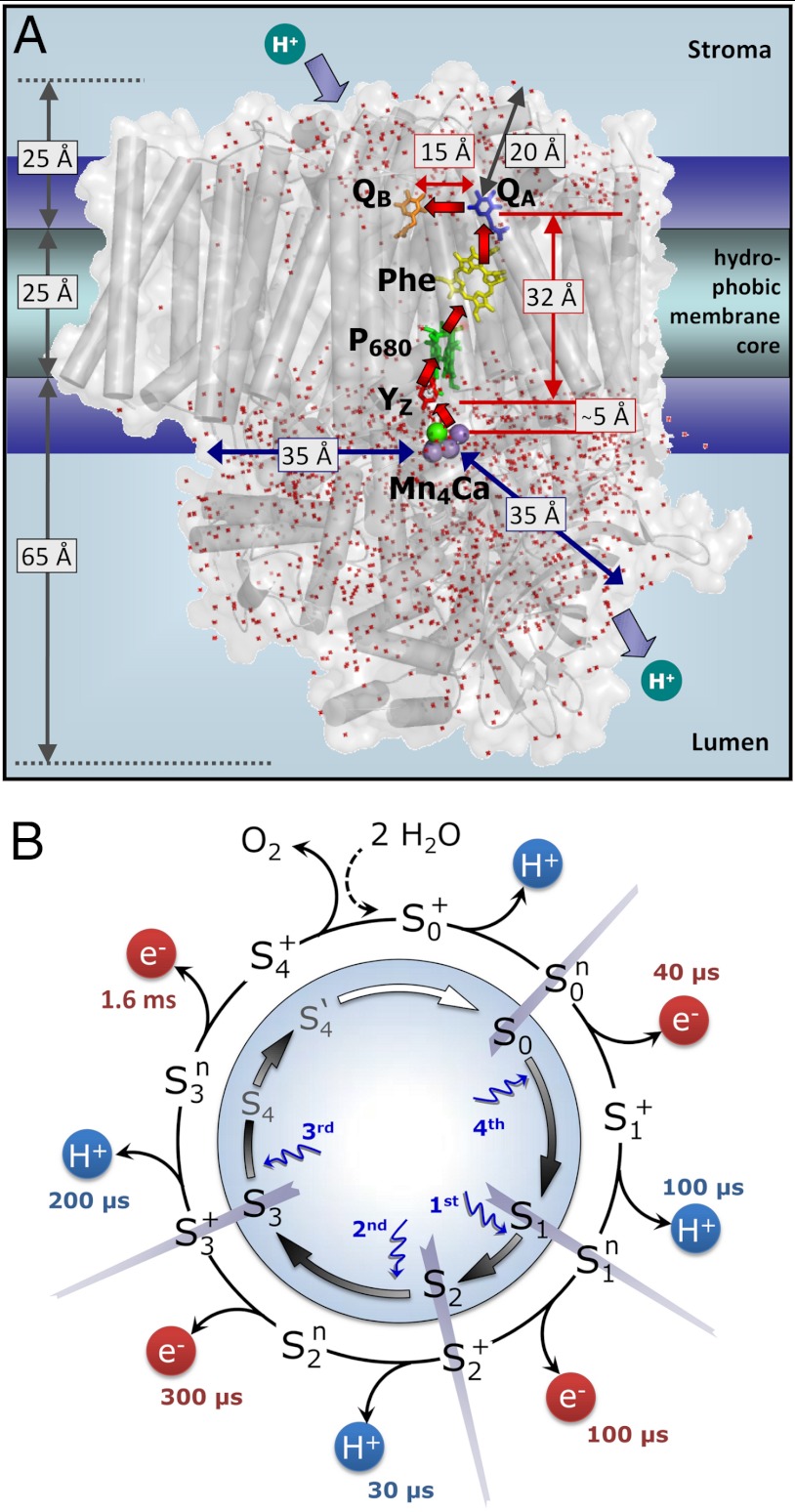
Fig. 1.
Photosystem II (A) and reaction cycle of water oxidation (B). In A, crucial redox cofactors and dimensions of the PSII complex are shown (15). Red arrows connect redox cofactors of the ET chain, including the primary electron donor (P680), the primary pheophytin acceptor (Phe), the primary (QA) and secondary (QB) quinone acceptors, and, at the electron donor side, a redox-active tyrosine (YZ) and the Mn complex. Water molecules resolved in the crystallographic model (Protein Data Bank entry 3ARC; ref. 15) are shown as red dots; the indicated distances illustrate relevant dimensions. In B, the classical Kok model (16) (inner circle, including states S4 and S4′; ref. 22) is extended to describe both oxidation of the Mn complex by ET to the YZ radical and proton removal from the Mn complex or its ligand environment by long-distance proton transfer. Coupling of the ET step to local proton shifts is not covered by the shown framework model. The subscripts indicate the number of oxidation equivalents accumulated at the Mn complex; the superscripts indicate the charge relative to the dark-stable S1-state (+, positive; n, neutral). The proton release steps in the S0 → S1 and S2 → S3 transitions have not been tracked in time-resolved experiments before, but now these steps are detected in the PBD experiments; the indicated time constants result from the present study.