Table I.
Potencies of Xanthine Derivatives at Adenosine A1 and A2 Receptors in Nanomolar Concentration Unitsa,b
| compd | R | R1 | X |
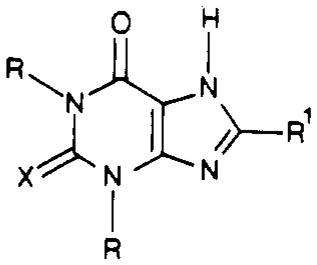
|
Ki(A2)/Ki(A1) | |
|---|---|---|---|---|---|---|
| Ki (A1 receptors) | Ki (A2 receptors) | |||||
| 1a | Me | H | O | 8470 ± 1490c | 25300 ± 2000a | 2.99 |
| 1b | Pr | H | O | 450 ± 25c | 5160 ± 590c | 11.5 |
| 2a | Me | cyclopentyl | O | 10.9 ± 0.9c | 1440 ± 70c | 133 |
| 2b | Pr | cyclopentyl | O | 0.9 ± 0.1 | 410 ± 40 | 455 |
| 13 | Me | cyclopentyl | S | 10.2 ± 1.5 | 1390 ± 88 | 136 |
| 14 | Pr | cyclopentyl | S | 0.655 ± 0.058 | 314 ± 62 | 479 |
| 15a | Me | 2-furyl | O | 350 ± 20c | 2780 ± 50c | 7.94 |
| 15b | Pr | 2-furyl | O | 37 ±6 | 640 ± 100 | 16.8 |
| 15c | Me | 2-furyl | S | 182 ± 36 | 4450 ± 420 | 10.6 |
| 15d | Pr | 2-furyl | S | 32 ± 5 | 594 ± 71 | 18.6 |
| 16 | Me | 3-furyl | O | 72.4 ± 3.7c | 984 ± 70c | 13.6 |
| 17 | Me | 2-thienyl | O | 233 ± 48.6 | 1630 ± 179 | 6.97 |
| 18 | Me | 3-thienyl | O | 152 ± 27 | 841 ± 109 | 5.53 |
| 19 | Pr | 2-thienyl | O | 16.1 ± 1.96 | 381 ± 27.7 | 23.6 |
| 20 | Pr | 3-thienyl | O | 10.0 ± 0.03 | 121 ± 18.2 | 12.1 |
| 21 | Me | 2-thienyl | S | 221 ± 43.3 | 1740 ± 153 | 7.87 |
| 22 | Pr | 2-thienyl | S | 35.1 ± 6.0 | >5000 | >142 |
| 23 | Me | phenyl | O | 86.0 ± 2.8c | 848 ± 115c | 9.85 |
| 24 | Et | phenyl | O | 44.5 ± 1.2c | 836 ± 73c | 19.4 |
| 25 | Pr | phenyl | O | 10.2 ± 2.6c | 180 ± 29c | 17.8 |
| 26a | Me | phenyl | S | 38 ± 6 | >7000 | >184 |
| 26b | Pr | phenyl | S | 16.1 ± 2 | 422 ± 33 | 26.1 |
| compd | R2 |
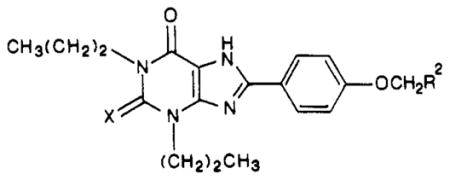
|
Ki (A2)/Ki (A1) | ||
|---|---|---|---|---|---|
| X | Ki (A1 receptors) | Ki (A2 receptors) | |||
| 27 | COOH | O | 58 ± 3 | 2200 ± 526 | 37.8 |
| 28 | COOH | S | 53.8 ± 7.1 | 315 ± 60.8 | 5.86 |
| 29 | COOEt | O | 42 ± 3 | >5000 | >119 |
| 30 | COOEt | S | 6.78 ± 0.64 | >5000 | >740 |
| 3 | CONH(CH2)2NH2 | O | 1.2 ± 0.5 | 63 ± 21 | 52.5 |
| 31 | CONH(CH2)2NH2 | S | 2.69 ± 0.77 | 26.3 ± 1.76 | 9.8 |
| 32 | CONH(CH2)2NHCH3 | O | 15.1 ± 1.6d | [9.3 ± 2.1]f | [0.62] |
| 33 | CONH(CH2)2NHCH3 | S | 2.4 ± 0.28 | 6.80 ± 1.36 | 2.8 |
| 34 | CONH(CH2)2N(CH3)2 | O | 2.8 ± 0.19d | 5.03 ± 0.54 | 1.8 |
| 35 | CONH(CH2)2N(CH3)2 | S | 2.55 ± 0.60 | 27.9 ± 7.5 | 11 |
| 36 | CON(CH3)(CH2)2N(CH3)2 | O | 0.93 ± 0.03d | 6.26 ± 0.25 | 6.7 |
| 37 | CON(CH3)(CH2)2N(CH3)2 | S | 2.57 ± 0.67 | 24.5 ± 8.4 | 9.5 |
| 38 |
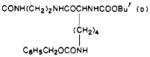
|
O | 12 | e | e |
| 39 |
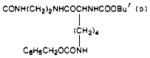
|
S | 84 | 870 | 10 |
| 40 |
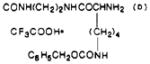
|
O | 6.4 ± 2.7 | 191 ± 13 | 30 |
| 41 |
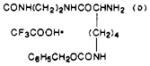
|
S | 8.9 | 322 ± 17 | 36 |
| 42 |
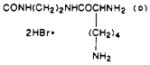
|
O | 0.87 ± 0.09 | 180 | 210 |
| 43 |
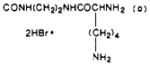
|
S | 13.0 ± 3.5 | 46.8 ± 9.4 | 3.6 |
| 44 |

|
O | 3.69 ± 0.71d | 207 ± 57d | 56 |
| 45 |
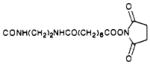
|
S | 33.5 | e | e |
| 46 |
|
O | 18.3 ± 3.0 | 147 ± 5 | 8.1 |
| 47 |
|
O | 16.2 ± 2.7 | 458 ± 34 | 28.3 |
| 48 |
|
O | 11.3 ± 1.5 | 116 ± 25 | 10.3 |
| 49 |
|
O | 7.44 ± 0.98 | 630 ± 160 | 85 |
| 50 |
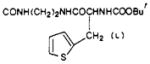
|
O | 17 ± 1.6 | e | e |
| 51 |
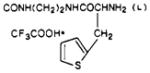
|
O | 1.3 ± 0.12 | e | e |
| compd | R | R1 |
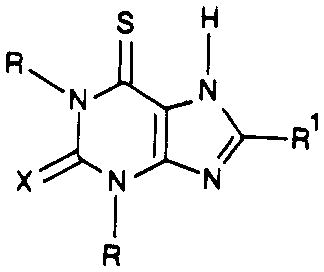
|
Ki (A2 receptors) | Ki(A2)/Ki(A1) | |
|---|---|---|---|---|---|---|
| X | Ki (A1 receptors) | |||||
| 52 | Me | cyclopentyl | S | 40.5 ± 6.6 | 11500 ± 628 | 285 |
| 53 | Pr | cyclopentyl | S | 4.87 ± 0.82 | 2780 ± 730 | 572 |
| 54 | Me | cyclopentyl | O | 202 ± 26 | 8980 ± 1300 | 44.4 |
| 55 | Pr | cyclopentyl | O | 15.5 ± 1.5 | 3360 ± 270 | 217 |
| 56 | Me | phenyl | O | 1380 ± 74 | 11300 ± 777 | 8.18 |
| 57 | Et | phenyl | O | 1010 ± 321 | 3510 ± 290 | 3.47 |
Ki value from a single determination run in triplicate or average of three ± SEM.
Inhibition of binding of [3H](phenylisopropyl)-adenosine to A1 receptors in rat cortical membranes and binding of [3H]-5′-(N-ethylcarbamoyl)adenosine to A2-adenosine receptors in rat striatal membranes was measured as described.18,19
Values taken from Bruns et al.18
Values taken from Jacobson et al.10
Not determined.
Kb for inhibition of 5′-(N-ethylcarbamoyl)adenosine-stimulated adenylate cyclase, in pheochromocytoma PC12 cell membranes.10b
