Abstract
Invasive alien species might benefit from phenotypic plasticity by being able to (i) maintain fitness in stressful environments (‘robust’), (ii) increase fitness in favourable environments (‘opportunistic’), or (iii) combine both abilities (‘robust and opportunistic’). Here, we applied this framework, for the first time, to an animal, the invasive slug, Arion lusitanicus, and tested (i) whether it has a more adaptive phenotypic plasticity compared with a congeneric native slug, Arion fuscus, and (ii) whether it is robust, opportunistic or both. During one year, we exposed specimens of both species to a range of temperatures along an altitudinal gradient (700–2400 m a.s.l.) and to high and low food levels, and we compared the responsiveness of two fitness traits: survival and egg production. During summer, the invasive species had a more adaptive phenotypic plasticity, and at high temperatures and low food levels, it survived better and produced more eggs than A. fuscus, representing the robust phenotype. During winter, A. lusitanicus displayed a less adaptive phenotype than A. fuscus. We show that the framework developed for plants is also very useful for a better mechanistic understanding of animal invasions. Warmer summers and milder winters might lead to an expansion of this invasive species to higher altitudes and enhance its spread in the lowlands, supporting the concern that global climate change will increase biological invasions.
Keywords: phenotypic plasticity, Jack-of-all-trades, molluscs, global warming, invasion, altitudinal gradient
1. Introduction
Invasive alien species [1] represent a major threat to biodiversity and cause economic costs [2,3]. The establishment and spread of invasive organisms outside their native range depends on both abiotic and biotic factors, such as invasive propagule pressure, the abiotic characteristics of the invaded ecosystem, the traits of the invaders, as well as ecological interactions between the invasive organisms and the native community [4]. As only a small fraction of all introduced organisms become invasive [4], understanding why and how some introduced plants and animals become successful invaders is paramount for maintaining native biodiversity [5].
Phenotypic plasticity in behavioural and fitness traits can be an important reason why some organisms become invasive [6–9]. It enhances ecological niche breadth [10–13] and thus allows an organism to establish populations in a broader range of environments. This idea dates back to the ‘general-purpose genotype’ of Baker [14], who suggested phenotypic plasticity as one characteristic of an ideal weed. According to a framework of phenotypic plasticity developed by Richards et al. [9], there are three scenarios of how an invader may benefit from phenotypic plasticity: first, in a Jack-of-all-trades scenario, the invader is able to maintain fitness in stressful situations (‘robust’). Second, in a Master-of-some situation, the invader is able to increase fitness in favourable environments (‘opportunistic’). Third, as a Jack-and-master, the invader has both of these abilities (‘robust and opportunistic’) [9]. This framework has been developed for a more mechanistic understanding of plant invasions but has, to our knowledge, never been applied to invasive animals. Generally, most evidence for phenotypic plasticity as an important trait of invasive organisms is derived from plants [15–19] with some studies also investigating vertebrates [20], aquatic organisms [7,21–27], entognatha [28,29] or insects [8,30,31]. Evidence from terrestrial molluscs, however, is largely lacking.
Temperature and nutrition are among the most important factors controlling survival and fecundity in terrestrial molluscs, and are therefore ideal for testing the framework developed by Richards et al. [9]. In general, lower temperatures retard gametogenesis, hatching time and growth of molluscs [32–34]. Sub-zero or high temperatures increase mortality unless the molluscs adapt physiologically, for example, by producing antifreeze agents [35–38]. Additionally, nutrition deprivation increases mortality and has a negative effect on the reproductive output of terrestrial molluscs by prolonging time until maturation and retarding growth rates of juveniles [34].
The effects of temperature and nutrition on fitness traits might interact. According to the metabolic theory of ecology, higher temperatures lead to higher metabolic rates in ectotherms, resulting in higher consumption and digestion rates [39]. McConnachie & Alexander [40], for example, showed, that gut passage time in lizards decreased significantly with increasing temperatures, leading to a faster nutrient assimilation rate at higher temperatures. Similarly, Naya et al. [41] showed for the brown garden snail (Cornu aspersum) that the intestine dry weight was increased under higher temperatures, and they related it to the simultaneously increased higher metabolic rate. The relationship between metabolic rates and temperatures might vary between species [42], and thus potentially leads to species-specific interactions between temperature and nutrition. To the best of our knowledge, however, this has not been investigated in terrestrial molluscs so far.
Here, we applied the framework developed by Richards et al. [9] to an invasive slug species, Arion lusitanicus (Mabille 1868), and tested (i) whether it has a more adaptive phenotypic plasticity in fitness traits under varying temperature and nutrition conditions compared with a native congeneric slug species, Arion fuscus (Mueller 1774), and (ii) which of the three scenarios it follows (robust, opportunistic or robust and opportunistic phenotype). During one year, we exposed specimens of both species to variation in two factors, namely to a range of temperatures along an altitudinal gradient (700–2400 m a.s.l.), and to high and low food levels, and compared the responsiveness of two fitness traits: survival and egg production.
2. Methods
(a). Study sites and study species
The study was conducted in the central Alps of Switzerland along an altitudinal gradient consisting of five sites that were evenly distributed between 700 and 2400 m a.s.l. (see pictures of the five sites and table S1 in the electronic supplementary material). Altitudinal gradients are among the most powerful ‘natural experiments’ for testing responses of organisms to different temperature conditions; with increasing altitude temperature drops while moisture remains constant [43]. To keep abiotic conditions other than altitude as consistent as possible (e.g. precipitation, exposition), all sites were situated along the same mountain slope, except the highest site, which was on the top of the closest higher mountain of the same massif.
Arion lusitanicus is one of the hundred most invasive alien species in Europe [44]. In central Europe, first records of A. lusitanicus date back to the 1970s [45–50]. Today, it occurs in all southern, central and northern European countries. In Switzerland, A. lusitanicus was first discovered in 1965, and since then has successfully invaded all lowland parts of the country [51] with highest number of observations between 560 and 1175 m a.s.l. (first and third quartiles; median: 840 m a.s.l.) [52]. Currently, in all European countries, an altitudinal expansion to higher altitudes is occurring [53] with specimens found in Switzerland already up to 2000 m a.s.l. [52]. Arion lusitanicus is 7–15 cm in size, polyphagous and it lives in various habitats, such as forests, gardens and agricultural habitats. Time of reproduction lasts from August to October. Hatchlings either emerge three weeks after egg-laying or they overwinter [54–56]. The lifespan lasts 12–13 months [54].
Arion fuscus is native to Europe and comprised a cryptic species complex of at least two distinct species, Arion subfuscus s. s. (based on the DNA analysis of the neotype) and A. fuscus [57]. Both species have been introduced elsewhere, namely North America [58–60], where A. subfuscus is invasive and expanding its range [61], whereas A. fuscus is not [58]. In Switzerland, A. fuscus is common but locally less abundant than the invasive A. lusitanicus, and it has a wide altitudinal range occurring up to 2500 m a.s.l. [51], with the highest occurrence by density between 540 and 1690 m a.s.l. (first and third quartiles; median: 1050 m a.s.l.). We have deliberately chosen a congeneric species that is very common and has a wide altitudinal range in order to have an appropriate control. Further, A. fuscus is a medium-sized slug species of 5–7 cm, closely related to A. lusitanicus, has a similar life cycle, and similar requirements regarding food and habitat [54]. To the best of our knowledge, nothing is known about ecological interactions between the two species, such as competition for the same resource.
At all sites, specimens of the invasive slug A. lusitanicus and the non-invasive slug A. fuscus were kept for 1 year in pots (22 cm in diameter and 21 cm in height), and their fitness traits (survival and reproduction) were assessed.
(b). Experimental set-up
From April to June 2009, 300 specimens of A. lusitanicus and A. fuscus were collected from 28 different locations in the Swiss lowlands (550–1100 m a.s.l.). Because it is not possible to unambiguously distinguish between A. fuscus and A. subfuscus when they are still alive, we relied on the distribution range and occurrence probability of the two species: A. fuscus is common in Switzerland, whereas A. subfuscus is very rare, with only 12 specimens being reported during the last 40 years, mainly from locations above 1700 m a.s.l. [52]. This might be partly due to the fact that the cryptic species complex was only discovered in 2004 [57]. Because we sampled all specimens below 1100 m a.s.l., we assume, however, to have sampled A. fuscus. Until the start of the experiment, the slugs were kept in a climatic chamber (d/n: 14/10 h, 18/14°C) and fed oat flakes.
Between 3 and 16 June 2009, specimens of each species were randomly distributed among the five altitudinal sites (60 of each species at each site) and kept in plastic pots. To ensure reproduction, four specimens of each species were kept together in the same pot. Dead individuals were not replaced so as to prevent bias that could result from introduction of new specimens from the lowlands. Thus, at each altitudinal site, a total of 15 pots per species were established.
The pots were covered with a gauze sheet to prevent slugs from escaping. A circular vegetation core (20 cm in diameter and approx. 10 cm in height) was taken from the surrounding grassland and put into the pots to establish a natural environment and to provide basic food for the slugs. In general, herbs make up the largest fraction of their food, followed by fungi, animals and soil [62]. The percentage of herbs of each vegetation core was estimated visually and included as a covariate in the statistical analyses. To establish variation in food levels (low versus high), seven randomly selected pots per site and species were supplemented weekly with cucumber and white mushrooms. Both are commonly used for slug breeding [63]. All plastic containers were placed in partial shade with full access to precipitation. At each site, temperature was measured by means of eight data loggers (Hygrochron iButtons, Maxim, Sunnyvale, CA), of which half were placed beneath the vegetation core inside randomly selected pots, while the remainder were placed on the ground outside the pots. Temperature was measured at 12.00 each day.
(c). Survival and reproduction
The experimental summer period lasted from June to August 2009 (figure 1). Survival was assessed by counting the number of live slugs in each pot at the beginning and end of the period. To assess reproductive output, the containers were searched weekly for egg clutches. Egg clutches were transferred to smaller clay pots filled with cotton wool (7 cm in diameter and 6 cm in height). To estimate the number of eggs, the weight of each clutch was divided by the mean weight of eggs. To account for varying egg size with altitude [64], the mean egg weight was calculated for each site and species from a subset of weighed egg clutches, of which all eggs were counted. The number of subsets analysed per site and species varied according to the total number available. In order to account for species-specific differences in body size, all specimens were weighed weekly. The experimental winter period started in October 2009 and lasted until spring 2010, depending on the snowmelt at the different altitudinal sites (figure 1). At the beginning of the experimental winter period, four surviving adults of the same species and site were packed together with a vegetation core into a fine gauze sheet and tightened with a string. Juveniles and eggs were packed in the same way, with 30 individuals per gauze sheet. In spring, surviving adults and juveniles, and juveniles hatched over winter, were counted.
Figure 1.
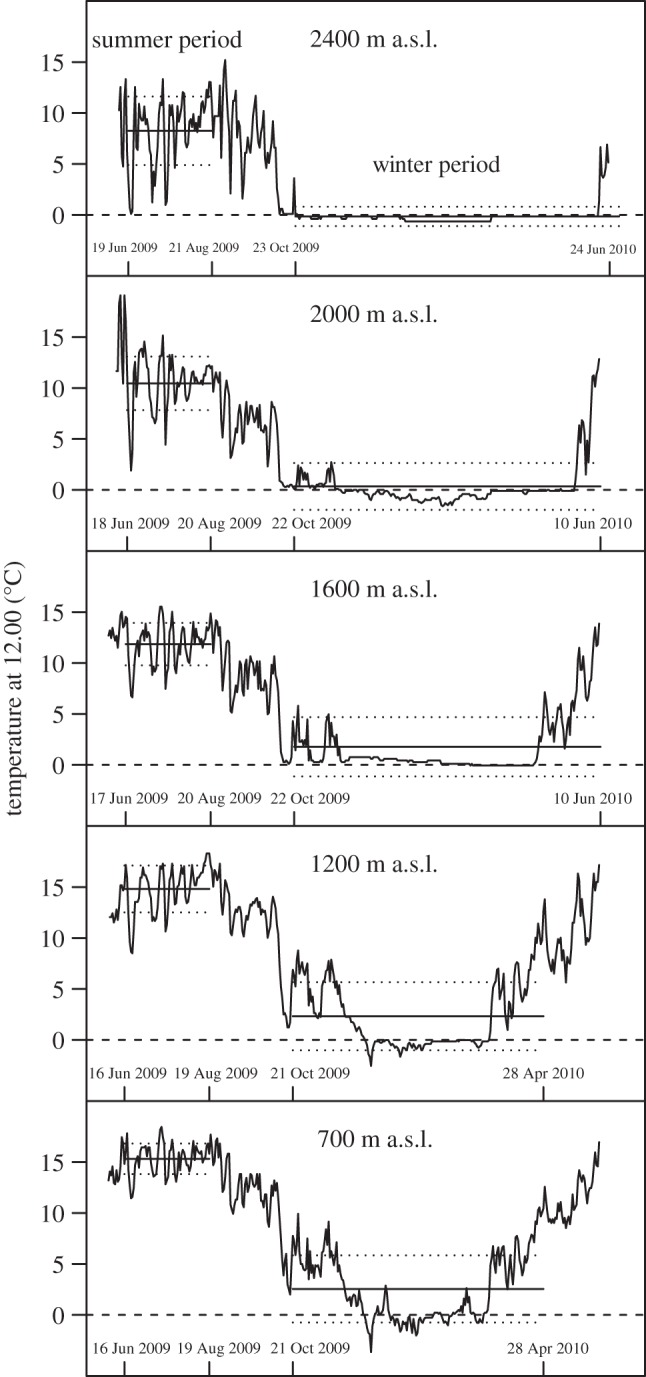
Daily mean temperature inside the pot (n = 4 per altitude) at 12.00 over the course of 1 year. Mean temperature (solid line) ± s.d. (dotted line) during the experimental summer period and winter period are shown.
(d). Statistical analyses
Differences in temperature between the altitudinal sites were analysed with a Kruskal–Wallis rank sum test using data from inside the pots, for the summer and winter experimental period separately. Differences between the mean temperature inside and outside the pots were analysed using a paired Student's t-test.
Because we did not replace specimens that died during the experiment, we first analysed whether reproduction and slug density in the pot were related. To do so, we used a generalized linear model (GLM) with survival as the response and number of eggs as the dependent variable, assuming a quasi-binomial error distribution.
Differences in reproductive output were analysed with a GLM assuming a quasi-Poisson error distribution. The number of eggs laid at the end of the experimental summer period was the dependent variable, with site (five levels: 700, 1200, 1600, 2000 and 2400 m a.s.l.), species (two levels: A. lusitanicus and A. fuscus) and food supplementation (two levels: weekly versus no food supplementation) as fixed factors, and herbs (percentage of herbs of the vegetation core) and weight (mean weight of the slugs within one pot over the course of the experiment) as covariates. The two covariates were first entered in the full model, and the minimal adequate model was determined by stepwise deletion of non-significant interactions and variables [65]. Significances of the minimal adequate model were calculated with an analysis of deviance using F-tests, terms added sequentially first to last [65]. If the interaction between site and species, and food supplementation and species, respectively, were significant, post hoc tests were run using a subset of the data, including all variables of the minimal adequate model, and assuming quasi-Poisson error distribution.
We measured the proportion of slugs surviving at the end of the experiment of the respective period (number of surviving individuals at the end of the period divided by the number of individuals at the beginning of the period). To analyse the summer, survival GLMs using a quasi-binomial error structure were fitted. In a first step, the full model with all interactions was fitted which included survival as dependent variable and species (two levels; see above), site (five levels; see above), food supplementation (two levels; see above), reproduction (two levels: pot with slugs that have laid eggs; pot without reproducing slugs) as fixed factors, and herbs (see above) and weight (see above) as covariates. Similar to the analyses of the reproductive output, the minimal adequate model was searched by stepwise deletion of non-significant factors and interactions, and significances were calculated with a sequential analysis of deviance [65]. Within factor level, differences of the significant interactions were run in the same way as described for the reproduction data (see above).
Winter survival was analysed separately for the adults, juveniles and eggs. Egg survival was determined by the proportion of juveniles hatched from the eggs in spring. The models included survival as dependent variable, and species (two levels; see above) and site (five levels; see above) as explanatory variables, and significances were calculated with a sequential analysis of deviance [65].
All statistical analyses were carried out using R v. 2.11.1 [66], and all data used are available as electronic supplementary material, tables S2–S5.
3. Results
(a). Temperature
Mean temperature varied significantly between the sites: during the experimental periods in summer and winter, the mean temperature inside the pots decreased with increasing elevation (figure 1; summer: K4,318 = 271.19, p < 2.2 × 10−16; winter: K4,1084 = 557.77, p < 2.2 × 10−16). During winter, the number of days below zero (0 ± 0.5°C) differed between sites with 24 d (700 m a.s.l.), 27 d (1200 m a.s.l.), 0 d (1600 m a.s.l.), 67 d (2000 m a.s.l.), 65 d (2400 m a.s.l.). At all altitudes, the temperature was significantly lower outside the pot: mean ± s.e. = −0.66 ± 0.1°C; 700 m a.s.l.: 95% CI (−0.66°C, −0.49°C), t385 = −13.03, p < 2.2 × 10−16; 1200 m a.s.l.: 95% CI (−0.61°C, −0.38°C), t384 = −8.16, p = 4.76 × 10−15; 1600 m a.s.l.: 95% CI (−0.24°C, −0.01°C), t376 = −2.05, p = 0.041; 2000 m a.s.l.: 95% CI (−0.69° C, −0.51°C), t369 = −13.45, p = 2.2 × 10−16; 2400 m a.s.l.: 95% CI (−0.83°C, −0.42°C), t377 = −6.01, p = 4.23 × 10−9).
(b). Reproduction
To estimate mean egg weight per site and species, a total of 38 clusters and 993 eggs were analysed, resulting in the following means ± s.e.: A. lusitanicus: 700 m a.s.l.: 0.026 ± 0.001; 1200 m a.s.l.: 0.024 ± 0.001; 1600 m a.s.l.: 0.025 (n = 1); A. fuscus: 700 m a.s.l.: 0.012 ± 0.001; 1200 m a.s.l.: 0.013 ± 0.001; 1600 m a.s.l.: 0.016 ± 0.002; 2000 m a.s.l.: 0.019 (n = 1). There was no relationship between number of eggs deposited and survival of the slugs (slope estimate ± s.e. = −0.002 ± 0.001, t1,137 = −1.315, p = 0.191). Significantly more eggs were produced when food was supplemented, and the number of eggs produced differed between sites, with more eggs produced at lower sites (table 1 and figure 2). There was a significant interaction between site and species, with the invasive A. lusitanicus producing significantly more eggs at higher altitudes (table 1 and figure 2). After accounting for the effect of food supplementation and site, the overall number of eggs produced did not differ between species (table 1 and figure 2). The effect of food supplementation, however, differed between species: A. fuscus produced no eggs when no food was supplemented, whereas A. lusitanicus maintained egg production (table 1 and figure 2).
Table 1.
Analysis of deviance of food supplementation (weekly versus no food supplemented), site (five altitudinal sites) and species (invasive A. lusitanicus versus congeneric and native A. fuscus) on the number of eggs produced, assuming a quasi-Poisson error distribution. Compared with A. fuscus, the invasive A. lusitanicus produced significantly more eggs when no food was supplemented (post hoc test; F1,74 = 29.32, p < 0.001) at the three highest sites (post hoc tests; 1600 m a.s.l.: F1,27 = 6.53, p = 0.019; 2000 m a.s.l.: F1,27 = 12.01, p = 0.002; 2400 m a.s.l.: F1,27 = 5.15, p = 0.032).
| d.f. | residual d.f. | residual deviance | F | p | |
|---|---|---|---|---|---|
| null model | 149 | 21 857.7 | |||
| food supplementation | 1 | 148 | 17 988.1 | 48.96 | <0.001 |
| site | 4 | 144 | 11 493.2 | 20.54 | <0.001 |
| species | 1 | 143 | 11 327.8 | 2.09 | 0.150 |
| site : species | 4 | 139 | 10 222.2 | 3.50 | 0.009 |
| food supplementation : species | 1 | 138 | 9097.3 | 14.23 | <0.001 |
Figure 2.
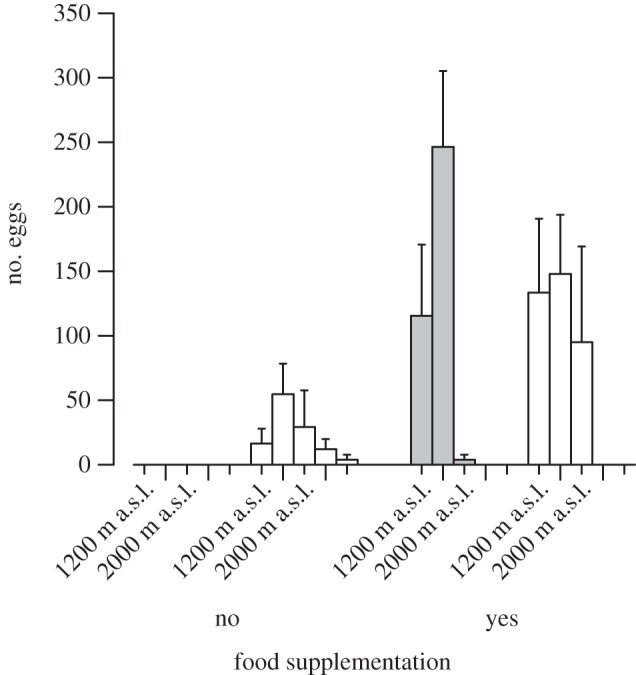
Mean (± s.e.) number of eggs laid by the invasive A. lusitanicus (white) and the congeneric and native Arion fuscus (grey) across the five altitudinal sites (700, 1200, 1600, 2000, 2400 m a.s.l.), and for the pots with and without food supplemented. Compared with A. fuscus, A. lusitanicus produced significantly more eggs when no food was supplemented and at the three highest sites (table 1).
(c). Survival during summer
Overall, food supplementation did not influence the survival of the two slug species (table 2). The survival varied significantly between sites and species, with a higher survival of the invasive A. lusitanicus (table 2 and figure 3). Only at the three lowest sites, however, A. lusitanicus had a significantly higher survival compared with A. fuscus (figure 3), explaining the significant interaction between site and species (table 2). Parameter estimates of the minimal adequate model showed a negative relationship between mean weight of the two slug species and survival (mean ± s.e. = −0.645 ± 0.264, t = −2.439, p = 0.016), and a positive relationship between percentage of herbs and survival of A. fuscus in the pots without food supplementation (mean ± s.e. = 0.061 ± 0.264, t = 3.100, p = 0.002). The latter relationship was significantly reduced for A. lusitanicus (mean ± s.e. = −0.050 ± 0.020, t = −2.442, p = 0.016; table 2). It was also significantly reduced (for both species) when food was supplemented to the pots (mean ± s.e. = −0.048 ± 0.020, t = −2.392, p = 0.018; table 2).
Table 2.
Analysis of deviance of food supplementation (weekly versus no food supplemented), site (five altitudinal sites), and species (invasive A. lusitanicus versus congeneric and native A. fuscus), and the two covariates herbs (percentage of herbs) and weight (mean weight of the slugs within one pot) on the summer survival of the two slugs species, assuming a quasi-binomial error distribution. For effect sizes and post hoc tests of the significant interaction between site and species, see figure 3.
| d.f. | residual d.f. | residual deviance | F | p | |
|---|---|---|---|---|---|
| null model | 138 | 375.76 | |||
| weight | 1 | 137 | 374.82 | 0.51 | 0.475 |
| herbs | 1 | 136 | 371.83 | 1.64 | 0.203 |
| food supplementation | 1 | 135 | 371.82 | 0.01 | 0.939 |
| site | 4 | 131 | 337.70 | 4.66 | 0.002 |
| species | 1 | 130 | 264.93 | 39.75 | <0.001 |
| herbs : food supplementation | 1 | 129 | 253.12 | 6.45 | 0.012 |
| herbs : species | 1 | 128 | 239.13 | 7.64 | 0.007 |
| site : species | 4 | 124 | 206.61 | 4.44 | 0.002 |
Figure 3.
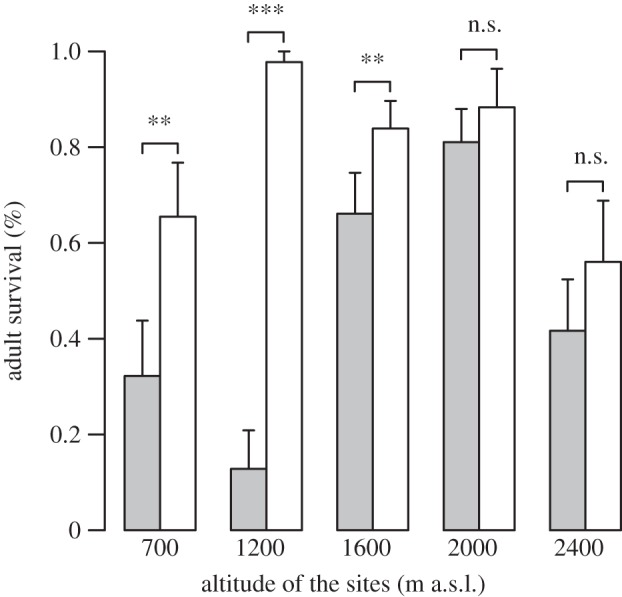
Mean (± s.e.) summer survival rate of the invasive Arion lusitanicus (white) and the congeneric and native Arion fuscus (grey) across the five altitudinal sites. There were significant differences between sites and species, and there was a significant interaction between site and species (table 2). Post hoc tests show that A. lusitanicus had a significantly higher survival rate at the lower three sites compared with A. fuscus (**p < 0.05; ***p < 0.001; n.s.p > 0.05).
(d). Survival during winter
During winter, juveniles and adults of A. fuscus had a significantly higher survival than A. lusitanicus (tables 3 and 4). The survival of juveniles and adults of both species varied significantly between altitudes: only adult specimens of A. lusitanicus survived the winter, and only at 1600 m a.s.l. By contrast, adult specimens of A. fuscus survived at all sites up to 2000 m a.s.l., and juveniles up to 1600 m a.s.l., with decreasing survival with increasing altitude (table 4). While the hatching rate after the winter period differed significantly between species, differences between sites were negligible (tables 3 and 4).
Table 3.
Analysis of deviance of site (five altitudinal sites) and species (invasive Arion lusitanicus versus congeneric and native Arion fuscus) on the winter survival of adults, juveniles and eggs, assuming a quasi-binomial error distribution. Because the slugs did not reproduce at all sites during summer, the interaction between site and species could only be estimated for the adults (table 4).
| d.f. | residual d.f. | residual deviance | F | p | |
|---|---|---|---|---|---|
| adults | |||||
| null model | 36 | 126.1 | |||
| site | 4 | 32 | 96.0 | 4.29 | 0.008 |
| species | 1 | 31 | 86.2 | 5.59 | 0.025 |
| site : species | 2 | 29 | 67.3 | 5.39 | 0.010 |
| juveniles | |||||
| null model | 12 | 132.8 | |||
| site | 2 | 10 | 31.7 | 38.15 | <0.001 |
| species | 1 | 9 | 13.5 | 13.68 | 0.005 |
| eggs | |||||
| null model | 34 | 33.7 | |||
| site | 1 | 33 | 7.5 | 122.21 | <0.001 |
| species | 2 | 31 | 7.2 | 0.49 | 0.612 |
Table 4.
Mean (±s.e.) percentage of the winter survival of adults, juveniles and eggs of the invasive A. lusitanicus and the congeneric and native A. fuscus over the experimental winter period. Numbers in brackets represent the number of individuals/eggs that entered the winter period. The table shows the higher the sites, the more retarded reproduction due to colder temperatures.
| m a.s.l. |
Arion fuscus |
Arion lusitanicus |
||||
|---|---|---|---|---|---|---|
| adults | juveniles | eggs | adults | juveniles | eggs | |
| 2400 | n.a. (0) | n.a. (0) | n.a. (0) | 0 (7) | n.a. (0) | n.a. (0) |
| 2000 | 58 ± 19 (23) | n.a. (0) | n.a. (0) | 0 (32) | n.a. (0) | n.a. (0) |
| 1600 | 46 ± 18 (28) | 5 ± 1 (65) | 5 ± 1 (87) | 46 ± 14 (28) | n.a. (0) | 0 (34) |
| 1200 | n.a. (0) | 58 ± 8 (60) | n.a. (0) | 0 (16) | n.a. (0) | 0 (570) |
| 700 | 0 (3) | 9 ± 7 (81) | 3 ± 2 (120) | 0 (6) | 0 (162) | 0 (240) |
4. Discussion
The results show that the lowest and highest altitudinal sites were stressful to both slug species and resulted in a significantly lower summer survival compared with the other sites. However, the invasive A. lusitanicus had a significantly higher survival rate than the congeneric and native A. fuscus owing to the higher survival at lower sites. This is consistent with the Jack-of-all-trades scenario [9]. Food supplementation as well as herbs did not significantly affect the survival rates. Interestingly, there was a significant interaction between herbs and species, as well as between herbs and food supplementation, showing that survival was increased when food was supplemented in pots containing a low percentage of herbs, and that only the survival of A. fuscus was positively related to the amount of herbs in the pot. The latter shows that the invasive species A. lusitanicus can either cope with a food shortage better or depends less on herbs, which are the most valuable food resource for slugs [62], suggesting again a Jack-of-all-trades scenario. There was no interaction between site and food supplementation, probably due to the fact that food supplementation did not significantly affect summer survival rates.
Both slug species had a significantly lower reproductive output at higher altitudinal sites and when no food was supplemented. There were, however, significant interactions between site and species, as well as food supplementation and species, suggesting that the invasive species represents a Jack-of-all-trades also with respect to egg production: although A. lusitanicus maintained egg production without food supplementation, A. fuscus did not reproduce at all. Similarly, the invasive species reproduced at all altitudinal sites, whereas the congeneric control species reproduced only at the two lowest sites. There was no significant interaction between site and food supplementation. This might be related to a recent finding by Marshall et al. [67], who, counter to the predictions of the metabolic theory of ecology [39], found a decreased metabolic rate in water snails under warmer conditions. Similarly, the metabolic rate of the two species might not be increased under increased temperatures, and thus the food supplementation effect was independent of temperature.
Being a Jack-of-all-trades might partly explain why A. lusitanicus is so successful in colonizing new areas: as a slug, it is not very mobile and depends on external assistance for long-distance dispersal, for example, anthropogenic transport by cars [68]. This requires withstanding warm and dry conditions, and reproducing without high food levels. Being robust to hot weather periods characteristic to summer might explain the dominance of A. lusitanicus in the lowland, and given global warming it is expected to even become more invasive.
During winter, only A. lusitanicus survived in pots where the temperatures did not fall below zero. Similarly, Lee et al. [69] found that the elevational range margins of the invasive slug Deroceras panormitanum are set by its physiological tolerances to low temperature and salinity. Slugs have two main strategies for overwintering, namely freeze tolerance and freeze avoidance, either by taking refuge in a thermally buffered site or by extensive supercooling [35–38]. To our knowledge, nothing is known about the overwintering strategy of our two target species apart from the study of Slotsbo et al. [70], who tested freezing tolerance of different life states of A. lusitanicus in the laboratory. They show that juveniles and adults are freeze-tolerant to a certain point but generally poor supercoolers, and conclude that the winter survival of A. lusitanicus depends to a high degree on migration to habitats protected from low temperatures. Our results also suggest that in comparison with the control species the invasive species is not freeze-tolerant but rather avoids freezing. This was, however, only partly possible because the slugs were caged in the pots. Owing to our semi-natural field approach, we could not control for humidity that could have affected the survival of the slugs, especially during snowmelt. As all pots had the same exposure and shelter conditions, however, we regard this potential impact to be the same at all sites.
Global warming is expected to further enhance invasive species and increase their impact on the ecosystem [71–75]. This is probably also the case for the invasive slug species investigated here. First, as a Jack-of-all-trades, A. lusitanicus will be able to maintain fitness under increased temperatures in its current range. Second, our data strongly suggest that cold temperatures in winter are currently limiting the spread of A. lusitanicus to higher altitudes and most likely also latitudes. Thus, with the predicted temperature increase of 0.6°C to 4°C over the next 100 years, this limitation will be reduced [76]. On the other hand, a reduction in the winter survival owing to global warming may occur if the winter snow cover is reduced or lost, resulting in exposure to more severe air temperatures and increasing frequency of freeze–thaw cycles [77]. Our results support this mechanism by the fact that A. lusitanicus did not survive at the lower and warmer sites, but without constant snow cover. However, the currently very high density of A. lusitanicus in the lowlands proves that this species is able to cope with freeze–thaw cycles, most likely due to behavioural adaptations that were only partly possible.
In summary, we show that the framework developed for plants by Richards et al. [9] is also very useful for a better mechanistic understanding of animal invasions. The investigated invasive slug A. lusitanicus displayed a more adaptive phenotypic plasticity in fitness traits under varying temperature and nutrition condition than the congeneric and native control species. Thereby, it followed a Jack-of-all-trades scenario, representing a robust phenotype. The recent spread of A. lusitanicus to higher altitudes is most likely to be due to increasing temperatures, subsequent climatic warming and a further expansion of this species in the lowlands, as well as to alpine areas.
Acknowledgements
We thank anonymous reviewers for their very constructive comments. We thank Jörg Rüetschi for helpful information slug sampling and identification, Patrick Kuss, Hans Boss and the Alpengarten Schynige Platte for provision of the altitudinal sites, and the Jungfraubahnen for financial support.
References
- 1.Richardson D. M., Pyšek P., Rejmánek M., Barbour M. G., Panetta D. F., West C. J. 2000. Naturalization and invasion of alien plants: concepts and definitions. Divers. Distrib. 6, 93–107 10.1046/j.1472-4642.2000.00083.x (doi:10.1046/j.1472-4642.2000.00083.x) [DOI] [Google Scholar]
- 2.Millennium Ecosystem Assessment 2005. Ecosystems and human well-being. Washington, DC: Island Press [Google Scholar]
- 3.Pimentel D., Zuniga R., Morrison D. 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Economics 52, 273–288 10.1016/j.ecolecon.2004.10.002 (doi:10.1016/j.ecolecon.2004.10.002) [DOI] [Google Scholar]
- 4.Richardson D. M., Pysek P. 2006. Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geogr. 30, 409–431 10.1191/0309133306pp490pr (doi:10.1191/0309133306pp490pr) [DOI] [Google Scholar]
- 5.Srivastava D. S., Vellend M. 2005. Biodiversity-ecosystem function research: is it relevant to conservation? Annu. Rev. Ecol. Evol. Syst. 36, 267–294 10.1146/annurev.ecolsys.36.102003.152636 (doi:10.1146/annurev.ecolsys.36.102003.152636) [DOI] [Google Scholar]
- 6.Hanfling B., Kollmann J. 2002. An evolutionary perspective of biological invasions. Trends Ecol. Evol. 17, 545–546 10.1016/s0169-5347(02)02644-7 (doi:10.1016/s0169-5347(02)02644-7) [DOI] [Google Scholar]
- 7.Valiente A. G., Juanes F., Nunez P., Garcia-Vazquez E. 2010. Brown trout (Salmo trutta) invasiveness: plasticity in life-history is more important than genetic variability. Biol. Invasions 12, 451–462 10.1007/s10530-009-9450-3 (doi:10.1007/s10530-009-9450-3) [DOI] [Google Scholar]
- 8.Wilson E. E., Mullen L. M., Holway D. A. 2009. Life history plasticity magnifies the ecological effects of a social wasp invasion. Proc. Natl Acad. Sci. USA 106, 12 809–12 813 10.1073/pnas.0902979106 (doi:10.1073/pnas.0902979106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richards C. L., Bossdorf O., Muth N. Z., Gurevitch J., Pigliucci M. 2006. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 9, 981–993 10.1111/j.1461-0248.2006.00950.x (doi:10.1111/j.1461-0248.2006.00950.x) [DOI] [PubMed] [Google Scholar]
- 10.Van Valen L. 1965. Morphological variation and width of ecological niche. Am. Nat. 99, 377–390 10.1086/282379 (doi:10.1086/282379) [DOI] [Google Scholar]
- 11.Chevin L.-M., Lande R., Mace G. M. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. 10.1371/journal.pbio.1000357 (doi:10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seigel R. A., Ford N. B. 2001. Phenotypic plasticity in reproductive traits: geographical variation in plasticity in a viviparous snake. Funct. Ecol. 15, 36–42 10.1046/j.1365-2435.2001.00492.x (doi:10.1046/j.1365-2435.2001.00492.x) [DOI] [Google Scholar]
- 13.Sultan S. E. 2001. Phenotypic plasticity for fitness components in Polygonum species of contrasting ecological breadth. Ecology 82, 328–343 10.2307/2679863 (doi:10.2307/2679863) [DOI] [Google Scholar]
- 14.Baker H. G. 1965. Characteristics and modes of origin of weeds. In The genetics of colonizing species (eds Baker H. G., Stebbins G. L.), pp. 147–160 New York, NY: Academic Press [Google Scholar]
- 15.Daehler C. C. 2003. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 34, 183–211 10.1146/annurev.ecolsys.34.011802.132403 (doi:10.1146/annurev.ecolsys.34.011802.132403) [DOI] [Google Scholar]
- 16.Kaufman S. R., Smouse P. E. 2001. Comparing indigenous and introduced populations of Melaleuca quinquenervia (Cav.) Blake: response of seedlings to water and pH levels. Oecologia 127, 487–494 10.1007/s004420000621 (doi:10.1007/s004420000621) [DOI] [PubMed] [Google Scholar]
- 17.Sexton J. P., McKay J. K., Sala A. 2002. Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol. Appl. 12, 1652–1660 10.1890/1051-0761(2002)012[1652:pagdma]2.0.co;2 (doi:10.1890/1051-0761(2002)012[1652:pagdma]2.0.co;2) [DOI] [Google Scholar]
- 18.Parker I. M., Rodriguez J., Loik M. E. 2003. An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conserv. Biol. 17, 59–72 10.1046/j.1523-1739.2003.02019.x (doi:10.1046/j.1523-1739.2003.02019.x) [DOI] [Google Scholar]
- 19.Funk J. L. 2008. Differences in plasticity between invasive and native plants from a low resource environment. J. Ecol. 96, 1162–1173 10.1111/j.1365-2745.2008.01435.x (doi:10.1111/j.1365-2745.2008.01435.x) [DOI] [Google Scholar]
- 20.Duncan R. P., Blackburn T. M., Sol D. 2003. The ecology of bird introductions. Ann. Rev. Ecol. Evol. Syst. 34, 71–98 10.1146/annurev.ecolsys.34.011802.132353 (doi:10.1146/annurev.ecolsys.34.011802.132353) [DOI] [Google Scholar]
- 21.Tamburi N. E., Martin P. R. 2011. Effects of food availability on reproductive output, offspring quality and reproductive efficiency in the apple snail Pomacea canaliculata. Biol. Invasions 13, 2351–2360 10.1007/s10530-011-0047-2 (doi:10.1007/s10530-011-0047-2) [DOI] [Google Scholar]
- 22.Trussell G. C., Smith L. D. 2000. Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenojtypic change. Proc. Natl Acad. Sci. USA 97, 2123–2127 10.1073/pnas.040423397 (doi:10.1073/pnas.040423397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosecchi E., Thomas F., Crivelli A. J. 2001. Can life-history traits predict the fate of introduced species? A case study on two cyprinid fish in southern France. Freshw. Biol. 46, 845–853 10.1046/j.1365-2427.2001.00715.x (doi:10.1046/j.1365-2427.2001.00715.x) [DOI] [Google Scholar]
- 24.Dzialowski A. R., Lennon J. T., O'Brien W. J., Smith V. H. 2003. Predator-induced phenotypic plasticity in the exotic cladoceran Daphnia lumholtzi. Freshw. Biol. 48, 1593–1602 10.1046/j.1365-2427.2003.01111.x (doi:10.1046/j.1365-2427.2003.01111.x) [DOI] [Google Scholar]
- 25.Alonso A., Castro-Diez P. 2008. What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia 614, 107–116 10.1007/s10750-008-9529-3 (doi:10.1007/s10750-008-9529-3) [DOI] [Google Scholar]
- 26.Kingsley-Smith P. R., Harwell H. D., Kellogg M. L., Allen S. M., Allen S. K., Meritt D. W., Paynter K. T., Luckenbach M. W. 2009. Survival and growth of triploid Crassostrea virginica (Gmelin, 1791) and C. ariakensis (Fujita, 1913) in bottom environments of Chesapeake Bay: implications for an introduction. J. Shellfish Res. 28, 169–184 10.2983/035.028.0201 (doi:10.2983/035.028.0201) [DOI] [Google Scholar]
- 27.Piola R. F., Johnston E. L. 2009. Comparing differential tolerance of native and non-indigenous marine species to metal pollution using novel assay techniques. Environ. Pollut. 157, 2853–2864 10.1016/j.envpol.2009.04.007 (doi:10.1016/j.envpol.2009.04.007) [DOI] [PubMed] [Google Scholar]
- 28.Chown S. L., Slabber S., McGeoch M. A., Janion C., Leinaas H. P. 2007. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B 274, 2531–2537 10.1098/rspb.2007.0772 (doi:10.1098/rspb.2007.0772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slabber S., Worland M. R., Leinaas H. P., Chown S. L. 2007. Acclimation effects on thermal tolerances of springtails from sub-Antarctic Marion Island: indigenous and invasive species. J. Insect Physiol. 53, 113–125 10.1016/j.jinsphys.2006.10.010 (doi:10.1016/j.jinsphys.2006.10.010) [DOI] [PubMed] [Google Scholar]
- 30.Nyamukondiwa C., Kleynhans E., Terblanche J. S. 2010. Phenotypic plasticity of thermal tolerance contributes to the invasion potential of Mediterranean fruit flies (Ceratitis capitata). Ecol. Entomol. 35, 565–575 10.1111/j.1365-2311.2010.01215.x (doi:10.1111/j.1365-2311.2010.01215.x) [DOI] [Google Scholar]
- 31.Lombaert E., Malausa T., Devred R., Estoup A. 2008. Phenotypic variation in invasive and biocontrol populations of the harlequin ladybird, Harmonia axyridis. Biocontrol 53, 89–102 10.1007/s10526-007-9131-z (doi:10.1007/s10526-007-9131-z) [DOI] [Google Scholar]
- 32.Gomot de Vaufleury A. 2001. Regulation of growth and reproduction. In The biology of terrestrial molluscs (ed. Barker G. M.), pp. 331–355 Wallingford, UK: CABI [Google Scholar]
- 33.Hommay G., Kienlen J. C., Gertz C., Hill A. 2001. Growth and reproduction of the slug Limax valentianus (Ferussac, 1823) in experimental conditions. J. Molluscan Stud. 67, 191–207 10.1093/mollus/67.2.191 (doi:10.1093/mollus/67.2.191) [DOI] [Google Scholar]
- 34.Rollo C. D., Shibata D. M. 1991. Resilience, robustness, and plasticity in a terrestrial slug, with particular reference to food quality. Can. J. Zool. 69, 978–987 10.1139/z91-142 (doi:10.1139/z91-142) [DOI] [Google Scholar]
- 35.Ansart A., Aulne P. A., Madec L., Vernon P. 2008. Influence of temperature acclimation and gut content on the supercooling ability of the land snail Cornu aspersum. Comp. Biochem. Physiol. 150, 14–20 10.1016/j.cbpa.2008.02.013 (doi:10.1016/j.cbpa.2008.02.013) [DOI] [PubMed] [Google Scholar]
- 36.Cook R. T. 2004. The tolerance of the field slug Deroceras reliculatum to freezing temperatures. CryoLetters 25, 187–194 [PubMed] [Google Scholar]
- 37.Storey K. B., Storey J. M., Churchill T. A. 2007. Freezing and anoxia tolerance of slugs: a metabolic perspective. J. Comp. Physiol. 177, 833–840 10.1007/s00360-007-0179-y (doi:10.1007/s00360-007-0179-y) [DOI] [PubMed] [Google Scholar]
- 38.Udaka H., Goto S. G., Numata H. 2008. Effects of photoperiod and acclimation temperature on heat and cold tolerance in the terrestrial slug, Lehmannia valentiana (Pulmonata: Limacidae). Appl. Entomol. Zool. 43, 547–551 10.1303/aez.2008.547 (doi:10.1303/aez.2008.547) [DOI] [Google Scholar]
- 39.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 40.McConnachie S., Alexander G. J. 2004. The effect of temperature on digestive and assimilation efficiency, gut passage time and appetite in an ambush foraging lizard, Cordylus melanotus melanotus. J. Comp. Physiol. B 174, 99–105 10.1007/s00360-003-0393-1 (doi:10.1007/s00360-003-0393-1) [DOI] [PubMed] [Google Scholar]
- 41.Naya D. E., Catalan T., Artacho P., Diego Gaitan-Espitia J., Nespolo R. F. 2011. Exploring the functional association between physiological plasticity, climatic variability, and geographical latitude: lessons from land snails. Evol. Ecol. Res. 13, 647–659 [Google Scholar]
- 42.Burton T., Killen S. S., Armstrong J. D., Metcalfe N. B. 2011. What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc. R. Soc. B 278, 3465–3473 10.1098/rspb.2011.1778 (doi:10.1098/rspb.2011.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korner C. 2007. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 22, 569–574 10.1016/j.tree.2007.09.006 (doi:10.1016/j.tree.2007.09.006) [DOI] [PubMed] [Google Scholar]
- 44.Hulme P. E. 2009. Handbook of alien species in Europe. Dordrecht, The Netherlands: Springer [Google Scholar]
- 45.Davies S. M. 1987. Arion flagellus Collinge and A. lusitanicus Mabille in the British Isles: a morphological, biological and taxonomic investigation. J. Conch. 32, 339–354 [Google Scholar]
- 46.De Winter A. J. 1989. Arion lusitanicus Mabille in Nederland (Gastropoda, Pulmonata, Arionidae). Basteria 53, 49–51 [Google Scholar]
- 47.Reischütz P. L., Stojaspal F. 1972. Bemerkenswerte Mollusken aus Ostösterreich. Mitteilungen der Zoologischen Gesellschaft Braunau 13, 339–344 [Google Scholar]
- 48.Risch P., Backeljau T. 1989. On the occurrence of Arion lusitanicus Mabille, 1868 in Belgium (Mollusca, Pulmonata). Ann. Soc. R. Zool. Belg. 119, 25–38 [Google Scholar]
- 49.Schmid G. 1970. Arion lusitanicus in Deutschland. Int. J. Malacol. 100, 95–102 [Google Scholar]
- 50.Wiktor A. 1983. The slugs of Bulgaria (Arionidae, Milacidae, Limacidae, Agriolimacidae–Gastropoda Stylommatophora). Ann. Zool. 37, 71–206 [Google Scholar]
- 51.Turner H. 1998. Atlas der Mollusken der Schweiz und Liechtensteins. Neuchâtel, Switzerland: Centre Suisse de Cartographie de la Faune [Google Scholar]
- 52.CSFC 2011. Centre Suisse de Cartographie de la Faune. Neuchâtel, Switzerland: See www.cscf.ch. [Google Scholar]
- 53.Kerney M. P., Cameron R. A. D., Jungbluth J. H. 1983. Die Landschnecken Nord- und Mitteleuropas ein Bestimmungsbuch für Biologen und Naturfreunde. Hamburg/Berlin, Germany: Parey [Google Scholar]
- 54.Bogon K. 1990. Landschnecken biologie—oekologie—biotopschutz. Augsburg, Germany: Natur-Verlag [Google Scholar]
- 55.Grimm B. 2001. Life cycle and population density of the pest slug Arion lusitanicus Mabille (Mollusca: Pulmonata) on grassland. Malacologia 43, 25–32 [Google Scholar]
- 56.Kozlowski J. 2007. The distribution, biology, population dynamics and harmfulness of Arion lusitanicus Mabille, 1868 (Gastropoda: Pulmonata: Arionidae) in Poland. J. Plant Prot. Res. 47, 219–230 [Google Scholar]
- 57.Pinceel J., Jordaens K., Van Houtte N., De Winter A. J., Backeljau T. 2004. Molecular and morphological data reveal cryptic taxonomic diversity in the terrestrial slug complex Arion subfuscus/fuscus (Mollusca, Pulmonata, Arionidae) in continental north-west Europe. Biol. J. Linn. Soc. 83, 23–38 10.1111/j.1095-8312.2004.00368.x (doi:10.1111/j.1095-8312.2004.00368.x) [DOI] [Google Scholar]
- 58.Barr N. B., Cook A., Elder P., Molongoski J., Prasher D., Robinson D. G. 2009. Application of a DNA barcode using the 16S rRNA gene to diagnose pest Arion species in the USA. J. Molluscan Stud. 75, 187–191 10.1093/mollus/eyn047 (doi:10.1093/mollus/eyn047) [DOI] [Google Scholar]
- 59.Chichester L. F., Getz L. L. 1969. The zoogeography and ecology of arionid and limacid slugs introduced into northeastern North America. Malacologia 7, 313–346 [Google Scholar]
- 60.Chichester L. F., Getz L. L. 1973. The terrestrial slugs of northeastern North America. Sterkiana 51, 11–42 [Google Scholar]
- 61.Mc Donnell R. J., Rugman-Jones P., Backeljau T., Breugelmans K., Jordaens K., Stouthamer R., Paine T., Gormally M. 2011. Molecular identification of the exotic slug Arion subfuscus sensu stricto (Gastropoda: Pulmonata) in California, with comments on the source location of introduced populations. Biol. Invasions 13, 61–66 10.1007/s10530-010-9789-5 (doi:10.1007/s10530-010-9789-5) [DOI] [Google Scholar]
- 62.Barker G. M. 2001. The biology of terrestrial molluscs. Wallingford, UK: CABI [Google Scholar]
- 63.Ryser S., Rindlisbacher N., Gruebler M. U., Knop E. 2011. Differential survival rates in a declining and an invasive farmland gastropod species. Agric. Ecosyst. Environ. 144, 302–307 10.1016/j.agee.2011.08.005 (doi:10.1016/j.agee.2011.08.005) [DOI] [Google Scholar]
- 64.Baur A., Baur B. 1998. Altitudinal variation in size and composition of eggs in the land snail Arianta arbustorum. Can. J. Zool. 76, 2067–2074 10.1139/cjz-76-11-2067 (doi:10.1139/cjz-76-11-2067) [DOI] [Google Scholar]
- 65.Crawley M. J. 2009. The R book. Chichester, UK: Wiley [Google Scholar]
- 66.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 67.Marshall D. J., McQuaid C. D. 2011. Warming reduces metabolic rate in marine snails: adaptation to fluctuating high temperatures challenges the metabolic theory of ecology. Proc. R. Soc. B 278, 281–288 10.1098/rspb.2010.1414 (doi:10.1098/rspb.2010.1414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aubry S., Labaune C., Magnin F., Roche P., Kiss L. 2006. Active and passive dispersal of an invading land snail in Mediterranean France. J. Anim. Ecol. 75, 802–813 10.1111/j.1365-2656.2006.01100.x (doi:10.1111/j.1365-2656.2006.01100.x) [DOI] [PubMed] [Google Scholar]
- 69.Lee J. E., Janion C., Marais E., van Vuuren B. J., Chown S. L. 2009. Physiological tolerances account for range limits and abundance structure in an invasive slug. Proc. R. Soc. B 276, 1459–1468 10.1098/rspb.2008.1240 (doi:10.1098/rspb.2008.1240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slotsbo S., Hansen L. M., Holmstrup M. 2011. Low temperature survival in different life stages of the Iberian slug, Arion lusitanicus. Cryobiology 62, 68–73 10.1016/j.cryobiol.2010.12.005 (doi:10.1016/j.cryobiol.2010.12.005) [DOI] [PubMed] [Google Scholar]
- 71.Dukes J. S., Mooney H. A. 1999. Does global change increase the success of biological invaders? Trends Ecol. Evol. 14, 135–139 10.1016/s0169-5347(98)01554-7 (doi:10.1016/s0169-5347(98)01554-7) [DOI] [PubMed] [Google Scholar]
- 72.Walther G. R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J. M., Hoegh-Guldberg O., Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 10.1038/416389a (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 73.Thuiller W., Richardson D. M., Midgley G. F. 2007. Will climate change promote alien invasions? In Biological invasions (ed. Nentwig W.), pp. 197–211 Berlin, Germany: Springer [Google Scholar]
- 74.Vila M., Corbin J. D., Dukes J. S., Pino J., Smith S. D. 2007. Linking plant invasions to global environmental change. In Terrestrial ecosystems in a changing world (eds Canadell J., Pataki D., Pitelka L.), pp. 93–102 New York, NY: Springer [Google Scholar]
- 75.Hellmann J. J., Byers J. E., Bierwagen B. G., Dukes J. S. 2008. Five potential consequences of climate change for invasive species. Conserv. Biol. 22, 534–543 10.1111/j.1523-1739.2008.00951.x (doi:10.1111/j.1523-1739.2008.00951.x) [DOI] [PubMed] [Google Scholar]
- 76.IPCC 2007. IPCC fourth assessment report: climate change 2007. Cambridge, UK: IPCC [Google Scholar]
- 77.Bale J. S., Hayward S. A. L. 2010. Insect overwintering in a changing climate. J. Exp. Biol. 213, 980–994 10.1242/jeb.037911 (doi:10.1242/jeb.037911) [DOI] [PubMed] [Google Scholar]


