Abstract
Rationale
Opioid receptor agonists can enhance some effects of cannabinoid receptor agonists and cannabinoid receptor agonists can enhance some effects of opioid receptor agonists; however, the generality of these interactions is not established.
Objective
This study examined interactions between the discriminative stimulus and antinociceptive effects of μ opioid receptor agonists and Δ9-tetrahydrocannabinol (THC) in rhesus monkeys.
Results
Neither heroin nor morphine (i.v. or s.c.) altered the discriminative stimulus effects of THC in monkeys (n=5) discriminating 0.1 mg/kg THC i.v. In contrast, THC (s.c.) markedly attenuated the discriminative stimulus effect of morphine and heroin in non-dependent monkeys (n=4) discriminating 1.78 mg/kg morphine s.c. Doses of THC that attenuated the discriminative stimulus effects of morphine in non-dependent monkeys failed to modify the discriminative stimulus effects of morphine in morphine-dependent (5.6 mg/kg/12 hr) monkeys (n=4) discriminating 0.0178 mg/kg naltrexone s.c. THC also failed to modify the discriminative stimulus effects of naltrexone in morphine-dependent monkeys or the effects of midazolam in monkeys (n=4) discriminating 0.32 mg/kg midazolam s.c. Doses of THC (s.c.) that attenuated the discriminative stimulus effects of morphine in non-dependent monkeys enhanced the antinociceptive effects of morphine (s.c.) in non-dependent monkeys. While μ receptor agonists did not alter the discriminative stimulus effects of THC, in a context-dependent manner THC altered the effects of μ receptor agonists.
Conclusion
That the same doses of THC enhance, attenuate, or do not affect morphine, depending on the condition, suggests that attenuation of morphine by THC can result from perceptual masking rather than common pharmacodynamic mechanisms or pharmacokinetic interactions.
Keywords: opioid, THC, drug discrimination, rhesus monkey, perceptual masking, morphine, heroin, midazolam, naltrexone, antinociception
Cannabinoid receptor agonists and μ opioid receptor agonists have some effects in common (e.g. antinociception): drugs from both classes have important therapeutic effects and some are abused (e.g., marijuana and heroin). Cannabinoid and opioid systems and drugs interact under a broad range of conditions, from cellular (Rios et al. 2006) to behavioral (e.g. Haney 2007) measures of drug action. Understanding these interactions could be especially important for developing novel medications that might comprise both cannabinoid and opioid mechanisms and for understanding the factors that contribute to polydrug abuse.
Especially relevant to polydrug abuse are studies on the combined effects of opioids and cannabinoids on behavioral measures that are related to and predictive of abuse, including drug self administration and drug discrimination. For example, the opioid antagonist naltrexone decreased Δ9-tetrahydrocannabinol (THC)-induced conditioned place preference (Braida et al. 2004) and THC self administration in squirrel monkeys responding under a continuous reinforcement schedule (Justinova et al. 2004) whereas THC increased heroin self administration (number of injections and breakpoint) in rats responding under a progressive ratio schedule (Solinas et al. 2005). Conversely, the cannabinoid CB1 receptor antagonist SR-141716A decreased heroin self administration in rats responding under a progressive ratio schedule and either decreased (Navarro et al. 2001) or had no effect (Solinas et al. 2003) on heroin self administration in rats responding under a continuous reinforcement schedule. These findings suggest that interactions between cannabinoids and opioids vary depending on the conditions under which the drugs are studied (e.g. schedule of reinforcement).
Opioid agonists and antagonists also can modify the discriminative stimulus effects of THC. For example, the μ opioid receptor agonists heroin and morphine enhanced while the opioid receptor antagonist naltrexone attenuated the discriminative stimulus effects of THC in rats (Solinas and Goldberg 2005). Initial reports indicated that naltrexone did not modify the subjective effects of THC in marijuana users (Wachtel and de Wit 2000; Greenwald and Stitzer 2000); however, more recent studies showed that naltrexone can increase (Haney et al. 2003) or decrease (Haney 2007) the subjective effects of THC, depending on the history of marijuana use. Thus, the nature of interactions between cannabinoid and opioid receptor agonists and antagonists appears to vary markedly across conditions. While a number of studies have examined how opioid receptor agonists and antagonists modify the effects of cannabinoid receptor agonists, little is known regarding how cannabinoid receptor agonists modify the behavioral effects of opioid receptor agonists and antagonists; however, the antinociceptive effects of cannabinoid and opioid receptor agonists can interact in an additive or supra-additive manner (see Cichewicz, 2004 for review).
This study evaluated possible interactions between THC and other drugs in five different groups of monkeys (four drug discrimination procedures and one antinociception procedure) to test the generality of published studies showing enhancement of the effects of THC by μ opioid receptor agonists. Thus, THC was studied in combination with heroin or morphine in non-dependent monkeys discriminating THC and in non-dependent monkeys discriminating morphine, in combination with naltrexone or morphine in morphine-dependent monkeys discriminating naltrexone, and in combination with morphine in a warm-water tail withdrawal (i.e. antinociception) procedure in non-dependent monkeys. Because THC markedly attenuated the discriminative stimulus effects of morphine under some and not other conditions, while enhancing the antinociceptive effects of morphine, THC also was studied in monkeys discriminating the benzodiazepine midazolam to further test the pharmacologic specificity of the interactions obtained with morphine and THC.
METHODS
Subjects
Twenty-one adult rhesus monkeys (Macaca mulatta; seven male, fourteen female) were used and had received drugs from various classes in previous studies: five (three male, two female) for the THC discrimination study; four (one male, three female) for the naltrexone discrimination study; four (two male, two female) for the morphine discrimination study; four (four female) for the midazolam discrimination study; and four (one male, three female) for the antinociception study. Monkeys (4–9 kg) and were maintained at 95% of their free-feeding weight with monkey chow (Harlan Teklad High Protein Monkey Diet, Madison, Wisconsin, USA), fresh fruit and peanuts provided after daily sessions. All monkeys were individually housed on a 14/10-h light/dark cycle with unlimited access to water in the home cage. Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences) and with the Guidelines for the Care and Use of Mammals in Neuroscience and Biobehavioral Research (National Research Council, 2003).
Surgery
Monkeys in the THC discrimination study were prepared with chronic indwelling catheters (heparin-coated polyurethane; o.d., 1.68 mm; i.d., 1.02 mm; Instech Solomon, Plymouth Meeting, Pennsylvania, USA) under surgical procedures described previously (McMahon 2006). Briefly, monkeys received ketamine (10 mg/kg i.m.) followed by isoflurane (1.5–3.0%, inhaled via face mask) and a catheter (Vicryl; Ethicon Inc., Somerville, NJ) was inserted in and fastened to the jugular or femoral vein. The distal end of the catheter was passed s.c. to the midscapular region where it was attached to an s.c. vascular access port (Mida-cbas-c50; Instech Solomon, Plymouth Meeting, Pennsylvania, USA).
Apparatus
During sessions monkeys were seated in commercially available primate chairs (Model R001, Primate Products, Miami, Florida, USA). For drug discrimination studies the seated monkeys were placed in ventilated, sound-attenuating chambers equipped with two response levers and stimulus lights located above each lever. Feet were placed in shoes containing brass electrodes to which a brief (250 msec, 3 mA) electric shock could be delivered from a remote a.c. generator. Experiments were controlled and data recorded with a microprocessor and a commercially available interface (Med Associates Inc., St. Albans, Vermont, USA).
Behavioral procedures
Drug discrimination
For the discrimination studies, separate groups of monkeys were trained previously to discriminate between vehicle and the respective training drug (THC, morphine, naltrexone or midazolam). On some days monkeys received an injection of a training drug and they could receive a reinforcer by responding on one of two available levers; on other days monkeys received an injection of vehicle and could receive a reinforcer by responding on the other lever. On test days, they could receive a reinforcer by responding on either lever.
Five monkeys discriminated 0.1 mg/kg THC (i.v.) in sessions comprising a 15-min timeout, during which chambers were dark and responses had no programmed consequence, followed by a 5-min response period, during which illumination of red lights signaled a pending electric stimulus, which was scheduled to occur every 15 s. The correct lever was determined by an i.v. injection (e.g. left, vehicle; right, THC) during the first minute of the cycle; designation of correct levers varied among monkeys and remained the same for an individual throughout the study. During response periods, five consecutive responses (FR 5) on the correct lever extinguished red lights and postponed delivery of the scheduled electric stimulus for 40 s. Responding on the incorrect lever reset the response requirement on the correct lever. Response periods ended after 5 min or after the delivery of four electric stimuli, whichever occurred first. Vehicle training comprised administration of a vehicle or sham injection during the first minute of each of no more than eight cycles. THC training comprised administration of 0.1 mg/kg THC during the first minute of a cycle followed by vehicle or sham during the first minute of a second cycle; completion of the FR on the THC-associated lever was required to postpone electric stimuli during both cycles. On some training days, one to six vehicle or sham training cycles preceded administration of THC. Monkeys had previously satisfied the criteria for testing defined as 5 consecutive or 6 of 7 days in which at least 80% of the total responses occurred on the correct lever and fewer than 5 responses (one FR) occurred on the incorrect lever prior to completion of the FR on the correct lever. For this study, these criteria had to be satisfied for one drug and one vehicle training session immediately prior to each test. The type of training session preceding a test session varied nonsystematically. Test sessions were identical to training sessions except that 5 consecutive responses on either lever postponed the shock schedule and animals received increasing doses of THC, alone or in combination with heroin or morphine. Drugs were studied up to doses that occasioned greater than 80% responding on the THC-associated lever, resulted in delivery of an electric stimulus, or to a cumulative dose of 0.32 mg/kg THC. With the exception of one study when 10.0 mg/kg morphine was administered s.c., all drugs were administered i.v. through an s.c. access port. A single injection of morphine (3.2, 5.6, and 10 mg/kg, i.v. as well as 10 mg/kg s.c.) or heroin (0.32, 0.56, and 1.0 mg/kg, i.v.) was administered immediately prior to the first cycle of a test session with increasing doses of THC administered in subsequent cycles.
For monkeys discriminating morphine, naltrexone, or midazolam, cycles comprised a 10-min timeout, during which the chamber was dark and responses had no programmed consequence, followed by a 5-min response period, during which illumination of red lights signaled a pending electric stimulus. With the exceptions noted below, all other parameters for these discrimination procedures were as described above for monkeys discriminating THC.
In four monkeys discriminating 1.78 mg/kg (s.c.) of morphine, heroin and morphine were studied alone and with THC (s.c.). Four other monkeys were treated twice daily with morphine (5.6 mg/kg/12 hr, s.c.) for at least one year and discriminated 0.0178 mg/kg (s.c.) naltrexone under conditions that were in all other respects as described above. Daily sessions began 3 hr after the morning injection of morphine. When morphine treatment was temporarily suspended (27 hr), these monkeys responded on the naltrexone lever; that effect was reversed by subsequent administration of morphine. THC was studied both in combination with increasing doses of naltrexone (i.e., in monkeys that received morphine 3 hr prior to the session) and, on a different occasion, with increasing doses of morphine (in monkeys that were acutely deprived of morphine). Four other monkeys discriminated 0.32 mg/kg (s.c.) midazolam while responding under an FR 10 schedule of stimulus-shock termination. Other experimental parameters for these monkeys were as described above. THC (s.c.) was administered 1 hr prior to sessions.
Thermal antinociception
The warm-water, tail-withdrawal procedure is described in detail elsewhere (Li et al. 2007). Monkeys were seatedin chairs and the lower part (15 cm) of their shaved tail was immersed in a thermal flask containing40, 50, or 55° C water. Testing with different temperatures varied non-systematically among monkeys and across cycles. When a subject failed to remove its tail within 20 sec, the experimenter removed the tail from the water and a latency of 20 sec was recorded. Test sessions began with control (no drug) determinationsfor each temperature. Every 15 min (i.e., one cycle) tail withdrawal latencies were measured for all three temperatures with 1 min between determinations. The effects of THC (0.32 and 1.0 mg/kg, s.c.) were assessed alone, when administered immediately prior to the first of 8 cycles (120 min), and also in combination with increasing doses of morphine s.c. (THC was administered 1 hr prior to the first dose of morphine) administered during the first minute of consecutive cycles. A test was terminated for an individual when the maximum effect (20 sec) was obtained for 50° C.
Drugs
The compounds used included the following: morphine sulfate, naltrexone hydrochloride, heroin hydrochloride, the levo enantiomer of Δ9-THC (100 mg/ml in absolute ethanol) (The Research Technology Branch, National Institute on Drug Abuse, Rockville, Maryland, USA), and midazolam hydrochloride (Roche Pharma Inc., Manati, Puerto Rico). Midazolam was purchased as a commercially prepared solution (5 and 100 mg/ml) and diluted with saline. THC was dissolved in a 1:1:18 mixture of absolute ethanol, Emulphor-620 (Rhone-Poulenc Inc., Princeton, New Jersey, USA), and physiologic saline. All other drugs were dissolved with saline. Except for experiments in monkeys discriminating THC (see Results for details), drugs were administered s.c. in a volume of 0.2 to 1.0 ml, and doses were expressed as the forms indicated above.
Data Analyses
For drug discrimination studies, the following two dependent variables were measured: the percentage of responses on the drug-appropriate lever during the response period, calculated by dividing the number of responses on the drug-appropriate lever by the total number of responses on both levers and multiplying by 100; and the rate of responding during the response period, calculated by dividing the total number of responses made on both levers by the duration of the response period in seconds (excluding timeouts). The mean percentage of responses on the drug-appropriate lever ± 1 SEM and the mean rate of responding ± 1 SEM during test sessions were plotted as a function of dose. Potencies were obtained by estimating the dose required to generate 50% responding on the drug-appropriate lever (ED50), along with 95% confidence limits (95% CL), using linear regression. For the antinociception study, tail withdrawal latency was expressed as a percentage of the maximal possible effect (MPE) using the following formula: % MPE= [(test latency−control latency)/(20 sec − control latency)] ×100, where the control latency was defined as the average latency determined in the absence of drug. The MPE was calculated for each individual and then averaged to obtain a group mean. Potencies were obtained by estimating the dose required to produce 80% of the MPE (ED80) using linear regression. ED80 values were determined for the group data because the MPE was less than 80% for two monkeys (55° C) when 1.0 mg/kg THC was studied in combination with 1.0 mg/kg morphine (see Results).
Discriminative stimulus effects of drugs administered alone and in combination were analyzed by fitting straight lines to the individual dose-response data by means of GraphPad Prism version 5.0 for Windows (GraphPad Software, Inc., San Diego, California, USA), using the following equation: effect = slope x log(dose) + intercept. For these analyses, only the linear portion of the dose-effect curve was used, defined by doses producing 25 to 75% of the maximum possible effect, including not more than one dose producing less than 25% and not more than one dose producing more than 75% of the maximum possible effect. Other doses were excluded from the analyses. The slopes and intercepts of dose-response curves generated from each drug administered alone and in combination were compared with an F-ratio test; the drug combination was considered to be significantly different from the drug alone when the data sets could not be described with a single line. Similar analyses were used to examine all drug combinations. In cases where the all of the data collected were either less than 50% (i.e., discrimination) or 80% (i.e., antinociception), the available data on the linear part of the curve were used for extrapolating the slope and intercept.
RESULTS
THC discrimination
THC increased responding on the THC-associated lever with a dose of 0.1 mg/kg occasioning more than 90% drug-lever responding (Table 1; ED50 [95% CL] = 0.037 mg/kg [0.023, 0.050]). Acute injections of heroin or morphine occasioned responding exclusively on the vehicle-associated lever (i.e., 0% drug-lever responding); the same doses of heroin and morphine failed to alter the discriminative stimulus effects of THC (Table 1). The lines fitting the linear portion of the dose-response data for THC in the presence and absence of morphine or heroin could be fitted with a common slope and common intercept, indicating that neither morphine nor heroin modified this effect of THC.
Table 1.
Effects of morphine and heroin on the discriminative stimulus effects of THC in rhesus monkeys discriminating 0.1 mg/kg THC (i.v.).
| Training drug and dose (mg/kg) | THC-lever responding (%, mean±SEM) | Rate (responses/second) | |
|---|---|---|---|
| THC | |||
| Vehicle | 0±0 | 1.92±0.39 | |
| 0.0032 | 1.2±0.8 | 2.02±0.39 | |
| 0.001 | 4.8±2.5 | 1.64±0.43 | |
| 0.032 | 46.6±13.0 | 1.55±0.43 | |
| 0.1 | 95.2±2.2 | 1.55±0.36 | |
| 3.2 morphine | Vehicle | 0±0 | 1.64±0.26 |
| 0.0032 | 5.4±5.4 | 1.58±0.26 | |
| 0.001 | 3.6±2.5 | 1.36±0.13 | |
| 0.032 | 27.0±16.3 | 1.23±0.20 | |
| 0.1 | 82.1±9.7 | 1.00±0.14 | |
| 0.32 | 99.0±1.0 | 0.48±0.01 | |
| 5.6 morphine | Vehicle | 0±0 | 1.42±0.25 |
| 0.0032 | 9.7±5.9 | 1.00±0.23 | |
| 0.001 | 5.0±3.8 | 1.04±0.27 | |
| 0.032 | 45.0±15.9 | 1.13±0.21 | |
| 0.1 | 81.5±12.0 | 1.05±0.27 | |
| 0.32 | 100±0 | 0.32±0.09 | |
| 10.0 morphine | Vehicle | 0±0 | 0.84±0.26 |
| 0.0032 | 20.1±12.3 | 0.39±0.11 | |
| 0.001 | 39.0±15.8 | 0.43±0.05 | |
| 0.032 | 56.3±22.3 | 0.22±0.01 | |
| 0.1 | 100±0 | 0.11±0.01 | |
| 0.32 heroin | Vehicle | 0±0 | 1.60±0.35 |
| 0.0032 | 4.6±4.6 | 1.78±0.34 | |
| 0.001 | 15.6±9.7 | 1.61±0.39 | |
| 0.032 | 62.2±13.9 | 1.3±0.34 | |
| 0.1 | 91.8±8.23 | 1.3±0.34 | |
| 0.56 heroin | Vehicle | 0±0 | 1.57±0.29 |
| 0.0032 | 18.9±11.8 | 1.11±0.17 | |
| 0.001 | 23.3±13.9 | 1.16±0.17 | |
| 0.032 | 28.7±19.5 | 1.32±0.19 | |
| 0.1 | 90.4±9.6 | 1.30±0.28 |
In the presence of 0.32 or 0.56 mg/kg heroin (i.v.), the ED50 values for THC (95% CL) were 0.030 (0.019, 0.040) or 0.051 mg/kg (0.018, 0.084) mg/kg, respectively; these doses of heroin in combination with THC did not markedly affect rate of responding. THC was not studied in combination with 1.0 mg/kg of heroin because that dose of heroin alone eliminated responding (data not shown). In the presence of 3.2, 5.6 or 10 mg/kg morphine (i.v.), the ED50 values for THC (95% CL) were 0.056 (0.036, 0.076), 0.052 (0.019, 0.084) and 0.032 (0.009, 0.056) mg/kg, respectively. Similarly, the ED50 value for THC in monkeys that received 10.0 mg/kg of morphine s.c. was 0.026 (0.011, 0.041) mg/kg. As the dose of morphine studied with THC increased, rate of responding decreased.
Morphine discrimination
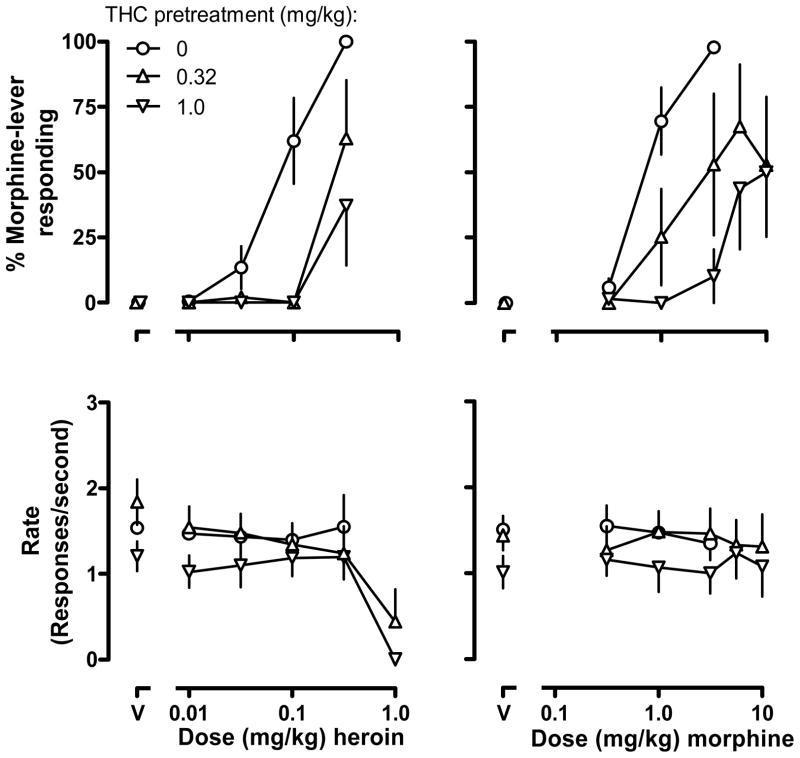
Heroin and morphine each increased responding on the morphine-associated lever with doses of 0.32 mg/kg heroin and 3.2 mg/kg morphine occasioning more than 90% drug-lever responding (open circles, upper panels, Fig. 1; heroin ED50 = 0.09 [0.05,0.13] mg/kg; morphine ED50 = 0.73 [0.66, 0.80] mg/kg). Acute injections of 0.32 or 1.0 mg/kg THC (“V” upper panels, Fig. 1) occasioned responding exclusively on the vehicle-associated lever. However, when administered as a pretreatment, each dose of THC attenuated the discriminative stimulus effects of heroin and morphine. For each opioid agonist, the lines fitting the linear portion of the dose-response data for heroin and morphine administered alone and in the presence of THC could be fitted with a common slope and different intercepts, indicting that THC shifted the heroin and morphine dose-response curves rightward. Although doses of 0.32 (triangles) and 1.0 (inverted triangles) mg/kg of THC attenuated the discriminative stimulus effects of heroin (left upper panel, Fig. 1), the magnitude of antagonism (shift in the dose-response curve) could not be estimated because the combination of THC and 1.0 mg/kg heroin markedly decreased responding and smaller doses of heroin administered in combination with THC did not occasion more than 80% responding on the drug-associated lever. Similarly, each of these doses of THC attenuated the discriminative stimulus effects of morphine (upper right panel, Fig. 1). Like heroin, the morphine dose-response curve was shifted downward and rightward by THC. For example, a dose of morphine (3.2 mg/kg) that occasioned responding exclusively on the drug-associated lever in all monkeys under control conditions, occasioned an average of less than 15% drug-lever responding in the presence of 1.0 mg/kg THC. These combinations of morphine and THC did not systematically alter rate of responding (lower right panel, Fig. 1).
Fig. 1.
Effects of THC (s.c.) on the discriminative stimulus and rate-altering effects of heroin (left) and morphine (right) in four rhesus monkeys discriminating 1.78 mg/kg morphine (s.c.). Ordinates: upper, average percentage of responses made on the morphine-associated lever (± SEM); lower, average rate of lever pressing (± SEM) expressed as responses per second. Abscissae: dose of drug in mg/kg body weight. “V” represents vehicle administered in the first test cycle.
Naltrexone discrimination
In monkeys treated twice daily with 5.6 mg/kg of morphine, naltrexone increased responding on the naltrexone-associated lever with a dose of 0.01 mg/kg occasioning 100% drug-lever responding (Table 2; ED50 = 0.005 [0.004, 0.006] mg/kg). When morphine treatment was temporarily (27 hr) discontinued, monkeys responded predominantly on the naltrexone-associated lever (Table 2); subsequent administration of morphine dose-dependently decreased responding on the naltrexone lever (ED50 = 1.21 [0.39, 2.02] mg/kg) Acute injections of 0.32 or 1.0 mg/kg THC neither substituted for naltrexone nor reversed naltrexone-lever responding in morphine-deprived monkeys. Moreover, neither of these doses of THC modified the discriminative stimulus effects of naltrexone in morphine-treated monkeys or the discriminative stimulus effects of morphine in morphine-deprived monkeys (Table 2). That is, the lines fitting the linear portions of the dose-response data for morphine and for naltrexone, each in the presence and absence of THC, could be fitted with a common slope and common intercept, indicating that THC did not modify these effects of morphine and naltrexone. In the presence of 0.32 and 1.0 mg/kg THC, the ED50 values for naltrexone were 0.006 (0.005, 0.006) and 0.005 (0.004, 0.006) mg/kg, respectively, and the ED50 values for morphine were 1.29 (0.59, 1.98) and 1.22 (0.47, 1.98) mg/kg, respectively. Combinations of THC with either naltrexone or morphine did not systematically alter rate of responding (Table 2)
Table 2.
Effects of THC on the discriminative stimulus effects of naltrexone in rhesus monkeys discriminating 0.0178 mg/kg naltrexone (s.c.) and on the discriminative stimulus effects of morphine in the same group of monkeys acutely deprived of morphine (27 h).
| Training drug and dose (mg/kg) | Naltrexone-lever responding (%, mean±SEM) | Rate (responses/second) | |
|---|---|---|---|
| Naltrexone | |||
| Vehicle | 0±0 | 2.02±0.46 | |
| 0.001 | 0±0 | 2.10±0.49 | |
| 0.0032 | 15.6±15.6 | 1.90±0.54 | |
| 0.01 | 97.5±2.5 | 1.68±0.40 | |
| 0.32 THC | Vehicle | 0±0 | 2.13±0.51 |
| 0.001 | 0±0 | 2.05±0.43 | |
| 0.0032 | 3.1±3.1 | 1.97±0.51 | |
| 0.01 | 96.7±2.6 | 1.41±0.42 | |
| 1.0 THC | Vehicle | 0±0 | 2.27±0.40 |
| 0.001 | 0±0 | 2.00±0.42 | |
| 0.0032 | 12.5±12.5 | 1.94±0.26 | |
| 0.01 | 99.5±0.5 | 1.39±0.45 | |
| Morphine | |||
| Vehicle | 97.0±1.9 | 1.46±0.43 | |
| 0.56 | 72.5±21.4 | 1.45±0.37 | |
| 1.0 | 44.5±21.6 | 1.53±0.35 | |
| 1.78 | 31.1±24.0 | 1.85±0.31 | |
| 3.2 | 0±0 | 1.53±0.42 | |
| 0.32 THC | Vehicle | 96.7±2.9 | 1.98±0.38 |
| 0.56 | 92.7±3.2 | 1.42±0.62 | |
| 1.0 | 38.9±17.3 | 1.45±0.36 | |
| 1.78 | 8.6±7.5 | 1.61±0.21 | |
| 3.2 | 4.9±4.2 | 1.61±0.22 | |
| 1.0 THC | Vehicle | 100±0 | 1.66±0.38 |
| 0.56 | 73.3±23.1 | 1.49±0.40 | |
| 1.0 | 62.8±27.3 | 1.21±0.37 | |
| 1.78 | 33.3±28.9 | 1.47±0.37 | |
| 3.2 | 0±0 | 1.40±0.37 |
Midazolam discrimination
Midazolam increased responding on the midazolam-associated lever with a dose of 0.32 mg/kg occasioning more than 90% responding on the drug-lever (Table 3; ED50 = 0.13 [0.05, 0.20] mg/kg). Acute injections of 0.32 or 1.0 mg/kg THC failed to substitute for midazolam and pretreatment with the same doses of THC failed to attenuate the discriminative stimulus effects of midazolam (Table 3). The lines fitting the linear portion of the dose-response data for midazolam in the presence and absence of THC could be fitted with a common slope and common intercept, indicating that THC did not modify this effect of midazolam. In the presence of 0.32 and 1.0 mg/kg THC, the ED50 values for midazolam were 0.07 (0.05, 0.08) and 0.11 (0.05, 0.18) mg/kg, respectively. The rate-decreasing effects of midazolam were enhanced by pretreatment with 1.0 mg/kg THC. A larger dose (1.78 mg/kg) of THC further decreased responding which precluded determination of a dose-response curve for midazolam in the presence of that dose of midazolam (data not shown).
Table 3.
Effects of THC on the discriminative stimulus effects of midazolam in rhesus monkeys discriminating 0.32 mg/kg midazolam (s.c.).
| Training drug and dose (mg/kg) | Naltrexone-lever responding (%, mean±SEM) | Rate (responses/second) | |
|---|---|---|---|
| Midazolam | |||
| Vehicle | 0±0 | 2.69±0.45 | |
| 0.01 | 0±0 | 2.36±0.47 | |
| 0.032 | 0±0 | 2.47±0.26 | |
| 0. 1 | 47.0±27.2 | 1.71±0.19 | |
| 0.32 | 93.5±3.8 | 1.14±0.16 | |
| 0.32 THC | Vehicle | 0±0 | 3.00±0.28 |
| 0.01 | 0±0 | 2.76±0.30 | |
| 0.032 | 0±0 | 2.67±0.24 | |
| 0. 1 | 88.9±7.4 | 1.36±0.33 | |
| 0.32 | 100±0 | 0.29† | |
| 1.0 THC | Vehicle | 0±0 | 2.66±0.35 |
| 0.01 | 0±0 | 2.27±0.35 | |
| 0.032 | 0±0 | 2.18±0.40 | |
| 0. 1 | 50.9±24.3 | 2.02±0.44 | |
| 0.32 | 98.9±1.1 | 1.82±0.34 |
Data from one monkey.
Antinociception
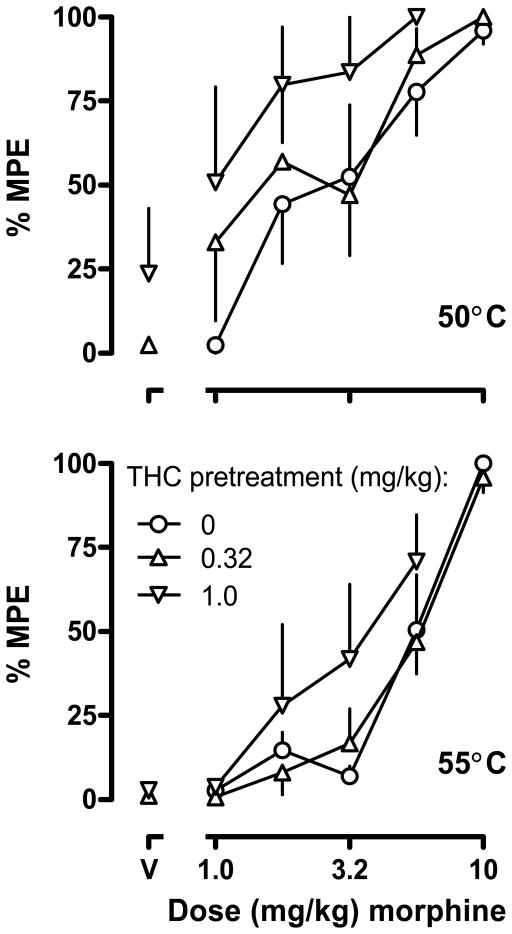
Under control conditions the average latency for monkeys to remove their tails (mean ± SEM, in seconds) from 40, 50 and 55 ° C water was 20 ± 0 (i.e., the maximum possible effect), 1.88 ± 0.68, and 1.07 ± 0.43, respectively. Morphine dose-dependently increased the latency for monkeys to remove their tails from 50° and 55° C water (open circles, Fig. 2; ED80 = 6.36 [3.81, 8.91] for 50° and ED80 = 7.92 [5.87, 9.27] for 55°). Acute injections of 0.32 mg/kg THC had no effect on tail withdrawal latency whereas a dose of 1.0 mg/kg slightly (24% MPE) increased latency at 50° C without affecting latency at 55° C (inverted triangles above “V”, Fig. 2). Pretreatment with THC enhanced the antinociceptive effects of morphine. Lines fitting the linear portion of the dose-response data obtained with morphine alone and in the presence of 1.0 mg/kg of THC at a temperature of 50° C could be fitted with a common slope but different intercepts, indicating that this dose of THC enhanced the antinociceptive effects of morphine. Other dose-response data (50 and 55° C) with morphine alone and with THC could be fitted with the same slope and intercept, indicated that under those conditions THC did not modify the antinociceptive effects of morphine. The ED80 values (50° C) for morphine administered with 1.0 mg/kg of THC was 2.42 mg/kg (upper panel, Fig. 2).
Fig. 2.
Effects of THC (s.c.) on the antinociceptive effects of morphine (s.c.) in four rhesus monkeys. Ordinates: average (± SEM) tail withdrawal latency from 50° (upper) and 55° C (lower) expressed as a percentage of the maximum (i.e., 20 sec) possible effect (%MPE). Abscissae: dose of drug in mg/kg body weight. “V” represents vehicle administered in the first test cycle.
DISCUSSION
It is well established that many of the behavioral effects of THC are mediated by cannabinoid CB1 receptors (e.g. Wiley JL 1999) and many of the behavioral effects of morphine are mediated by μ opioid receptors (e.g. Dykstra et al. 1988). While cannabinoids and opioids bind to different receptors, cannabinoid and μ opioid receptor agonists can affect common underlying neurochemical systems (e.g. dopamine; Gardner and Vorel 1998; Tanda and Goldberg 2003). A growing body of literature shows interactions between cannabinoid and opioid receptor agonists and antagonists, perhaps a result of actions on common underlying neurochemical systems. These interactions might be especially important for understanding polydrug abuse and the comorbidity of substance abuse with other pathologies (e.g. eating disorders) and for developing novel therapeutics that target both cannabinoid and opioid mechanisms. In rats discriminating THC, intracranial injection of the opioid peptide agonist β-endorphin or systemic administration of morphine or heroin enhanced, whereas administration of naltrexone attenuated, the discriminative stimulus effects of THC (Solinas et al. 2004; Solinas and Goldberg 2005); however, these interactions have not been unanimously confirmed. For example, in humans naltrexone attenuates, enhances, or has no effect the subjective and physiological effects of THC (Wachtel and de Wit 2000; Greenwald and Stitzer 2000; Haney et al. 2003; Haney 2007). These inconsistencies could be related to species, drug, dose, or history. For example, naltrexone attenuated THC intoxication in subjects who regularly smoked marijuana and enhanced THC intoxication in non-smokers (Haney et al. 2003; Haney 2007).
To test the generality of previous results and to further examine whether cannabinoid and μ opioid agonists interact in non-human primates, heroin and morphine were studied in rhesus monkeys discriminating THC. Up to the largest doses that could be studied, neither heroin nor morphine modified the discriminative stimulus effects of THC, contrasting enhancement of the discriminative stimulus effects of THC by μ opioid receptor agonists in rats (Solinas and Goldberg 2005). It is not clear what accounts for these differences.
A second study examined whether THC modified the discriminative stimulus effects μ opioid receptor agonists. If cannabinoids and opioids share effects on the same neurochemical systems, then THC might be expected to enhance the effects of μ opioid receptor agonists. In fact the opposite occurred; THC not only failed to enhance the discriminative stimulus effects of heroin and morphine, it markedly attenuated the effects of both drugs with two of four monkeys responding on the vehicle-associated lever up to a dose of morphine 3-fold larger than the dose that occasioned responding on the morphine-associated lever under control conditions. Collectively, these two studies failed to provide support for the view that the discriminative stimulus effects of cannabinoid and opioid receptor agonists interact in an additive manner.
A third study explored the generality of those findings by examining the same doses of THC and morphine in monkeys receiving 5.6 mg/kg of morphine twice daily and discriminating naltrexone; this procedure is very sensitive to and selective for μ opioid receptor agonists and antagonists (France et al. 1990; Li et al. 2007, 2008). In monkeys receiving morphine, μ opioid receptor antagonists occasion responding on the naltrexone-associated lever and precipitate signs of withdrawal (e.g. France et al. 1990). THC did not occasion responding on the naltrexone-associated lever and did not modify the discriminative stimulus effects of naltrexone, demonstrating a pharmacologic selectivity of THC in attenuating the discriminative stimulus effects of morphine (i.e., in untreated monkeys). When morphine treatment is temporarily discontinued, these monkeys respond on the naltrexone-associated lever and display signs of withdrawal; these effects are reversed by morphine and other μ receptor agonists (e.g. Gerak et al. 2003). The same doses of morphine that generalized to the training dose (1.78 mg/kg) in non-dependent monkeys (see above) reversed naltrexone-lever responding in morphine-dependent monkeys that were acutely deprived of morphine (compare Fig. 1 and Table 2). Doses of THC that attenuated the discriminative stimulus effects of morphine in non-dependent monkeys had no effect on the discriminative stimulus effects of the same doses of morphine in morphine-dependent, acutely-deprived monkeys. It is unlikely that different mechanisms (e.g., receptors) mediate the discriminative stimulus effects of morphine under these two conditions because, notwithstanding low-efficacy agonists, the same drugs (μ opioid receptor agonists) share effects with morphine and the affinity estimates for antagonists are the same in the two groups of monkeys (Li et al. 2008). That THC altered the discriminative stimulus effects of morphine only in non-dependent monkeys supports the view that drug history contributes to interactions between cannabinoids and opioids, just as modification the effects of THC in humans by naltrexone varied as a function of drug history (Haney et al. 2003; Haney 2007). It is possible that the pharmacokinetic profile of THC is different between untreated and morphine-dependent monkeys, although there is no clear evidence for this notion.
One test of whether drug history determines interactions between THC and μ opioid receptor agonists is to examine whether THC attenuates other behavioral effects of morphine in untreated monkeys. In non-dependent monkeys THC failed to attenuate the antinociceptive effects of morphine, with the morphine dose-response curves being shifted leftward by THC (e.g. additive interaction). It is unlikely that different mechanisms mediate the discriminative stimulus and antinociceptive effects of morphine because the same drugs (μ receptor agonists) share effects with morphine and the affinity estimates for antagonists are the same under the two conditions (Bowen et al. 2002). A positive interaction between the antinociceptive effects of cannabinoid and opioid receptor agonists in monkeys confirms previous findings in rodents (for reviews see Welch and Eades 1999; Cichewicz 2004) and suggests that the differential (e.g. opposite) effects obtained with THC and morphine across different measures and conditions is not due to pharmacodynamic or pharmacokinetic mechanisms. Further confirmation of the specificity of interactions between THC and μ opioid receptor agonists was that THC also failed to modify the discriminative stimulus effects of the benzodiazepine midazolam in non-dependent monkeys.
There are other examples from the drug discrimination literature of one drug attenuating the effects of a second drug in a manner that was not explained by known pharmacodynamic or pharmacokinetic mechanisms (Browne 1982; Colpaert 1977; Cook and Beardsley 2004; Doty et al. 1994; Gauvin and Young 1989; Gauvin et al. 1994; Kline and Young 1986; Koek et al. 2006; Negus et al. 1998; Nencini and Woolverton 1988; Platt et al. 1999; Young et al. 1992). These interactions can be distinguished from other interactions as follows: 1) they are not observed under all conditions, even with the same doses of the same drugs and using the same measure; 2) they are specific to discrimination; and 3) variations in training dose modify the interaction. Thus, drugs, like other stimuli (e.g. lights), might interact through perceptual masking (Colpaert 1977; but see Overton 1983) whereby one stimulus (drug) interferes with (masks) the detection of a second stimulus (drug).
First used to describe the audition of pure tones (Wegel and Lane 1924), perceptual masking has been applied to different stimuli (e.g. Breitmeyer and Ogmen 2000) and conditions (e.g. McClure 2001); this concept might be relevant for understanding polydrug use as well as the actions of drugs with multiple mechanisms of action, such as ethanol (Grant 1999), where a mosaic of stimulus effects is not mimicked by any one component of that stimulus. For example, amphetamine and ethanol can mask the effects of pentobarbital (Kline and Young 1986) and morphine (Gauvin and Young 1989), caffeine and buspirone can mask chlordiazepoxide (Gauvin et al. 1994), Δ9- and Δ8-THC can mask phencyclidine (Doty et al. 1994), and gamma-hydroxybutyrate and baclofen can mask flumazenil (Koek et al. 2006). In each case, neither pharmacodynamic nor pharmacokinetic mechanisms explained the interaction and the effects obtained with drug discrimination were not predicted by the combined effects of the same drugs under other conditions (i.e. interactions resulting from specific receptor mechanisms would be expected to yield similar results across conditions). Some factors that might contribute to masking include training dose (e.g. Gauvin and Young 1989; Mariathasan et al. 1996; Young et al. 1992), training history (e.g. Mariathasan et al. 1999; Stolerman and White 1996; Stolerman et al. 1999), and past or current drug treatment (e.g. Haney 2007). Other factors (e.g. drug class, stimulus salience) likely contribute to masking, although this topic has been studied relatively little, despite its potential importance for understanding drug combinations (therapeutic and non-therapeutic).
In summary, THC attenuated, enhanced, or did not affect the behavioral effects of morphine in rhesus monkeys. The situational (and not pharmacological) specificity of these interactions strongly suggests that something other than common pharmacodynamic mechanisms or pharmacokinetic factors account for these observations. These data underscore the necessity of evaluating the same drugs and drug combinations under different conditions in order to better understand the determinants of drug interactions.
Acknowledgments
This work was supported by USPHS Grants DA05018 (CPF), DA09157 (CPF), DA15468 (LRM), and DA19222 (LRM); CPF is supported by a Senior Scientist Award (DA17918). The authors have no other financial relationships with any organization that is relevant to the results presented in this manuscript.
The authors thank Christopher Cruz, Blake Harrington, Brandi Taylor, Armando Hernandez, and Ashley Varnon for expert technical assistance.
References
- Bowen CA, Fischer BD, Mello NK, Negus SS. Antagonism of the antinociceptive and discriminative stimulus effects of heroin and morphine by 3-methoxynaltrexone and naltrexone in rhesus monkeys. J Pharmacol Exp Ther. 2002;302:264–273. doi: 10.1124/jpet.302.1.264. [DOI] [PubMed] [Google Scholar]
- Braida D, Iosuè S, Pegorini S, Sala M. Δ9-Tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol. 2004;506:63–69. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ogmen H. Recent models and findings in visual backward masking: a comparison, review, and update. Percept Psychophys. 2000;62:1572–1595. doi: 10.3758/bf03212157. [DOI] [PubMed] [Google Scholar]
- Browne RG. Anxiolytics antagonize yohimbine’s discriminative stimulus properties. Psychopharmacology. 1981;74:245–249. doi: 10.1007/BF00427103. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74:1317–1324. doi: 10.1016/j.lfs.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Discriminative stimulus properties of benzodiazepines and barbiturates. In: Lal H, editor. Discriminative Stimulus Properties of Drugs. Plenum; New York: 1977. pp. 93–106. [Google Scholar]
- Cook CD, Beardsley PM. Modulation of the discriminative stimulus effects of mu opioid agonists in rats: II. Effects of dopamine D2/3 agonists. Behav Pharmacol. 2004;15:75–83. doi: 10.1097/00008877-200402000-00009. [DOI] [PubMed] [Google Scholar]
- Doty P, Dykstra LA, Picker MJ. Discriminative stimulus effects of phencyclidine: pharmacologically specific interactions with delta 9- and delta 8-tetrahydrocannabinol. Drug Alcohol Depend. 1994;35:151–158. doi: 10.1016/0376-8716(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Bertalmio AJ, Woods JH. Discriminative and analgesic effects of mu and kappa opioids: in vivo pA2 analysis. Psychopharmacol Ser. 1988;4:107–121. doi: 10.1007/978-3-642-73223-2_9. [DOI] [PubMed] [Google Scholar]
- France CP, de Costa BR, Jacobson AE, Rice KC, Woods JH. Apparent affinity of opioid antagonists in morphine-treated rhesus monkeys discriminating between saline and naltrexone. J Pharmacol Exp Ther. 1990;252:600–604. [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Peirce JM, Holloway FA. Perceptual masking of the chlordiazepoxide discriminative cue by both caffeine and buspirone. Pharmacol Biochem Behav. 1994;47:153–159. doi: 10.1016/0091-3057(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Young AM. Evidence for perceptual masking of the discriminative morphine stimulus. Psychopharmacology (Berl) 1989;98:212–221. doi: 10.1007/BF00444694. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Gauthier CR, France CP. Discriminative stimulus and antinociceptive effects of dihydroetorphine in rhesus monkeys. Psychopharmacology. 2003;166:351–359. doi: 10.1007/s00213-002-1268-y. [DOI] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- Haney M, Bisaga A, Foltin RW. Interaction between naltrexone and oral THC in heavy marijuana smokers. Psychopharmacology (Berl) 2003;166:77–85. doi: 10.1007/s00213-002-1279-8. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–1403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, Goldberg SR. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology (Berl) 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Kline FS, Young AM. Differential modification of pentobarbital stimulus control by d-amphetamine and ethanol. Pharmacol Biochem Behav. 1986;24:1305–1313. doi: 10.1016/0091-3057(86)90189-9. [DOI] [PubMed] [Google Scholar]
- Koek W, Carter LP, Wu H, Coop A, France CP. Discriminative stimulus effects of flumazenil: perceptual masking by baclofen, and lack of substitution with gamma-hydroxybutyrate and its precursors 1,4-butanediol and gamma-butyrolactone. Behav Pharmacol. 2006;17:239–247. doi: 10.1097/00008877-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Li JX, Becker GL, Traynor JR, Gong ZH, France CP. Thienorphine: receptor binding and behavioral effects in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:227–236. doi: 10.1124/jpet.106.113290. [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, France CP. Comparison of naltrexone, 6alpha-naltrexol, and 6beta-naltrexol in morphine-dependent and in nondependent rhesus monkeys. Psychopharmacology. 2008;195:479–486. doi: 10.1007/s00213-007-0914-9. [DOI] [PubMed] [Google Scholar]
- Mariathasan EA, Stolerman IP, White JA. AND and AND-OR drug mixture discriminations in rats: generalization to single drugs and drug mixtures. Psychopharmacology (Berl) 1999;143:54–63. doi: 10.1007/s002130050919. [DOI] [PubMed] [Google Scholar]
- Mariathasan EA, White JA, Stolerman IP. Training dose as a decisive factor for discrimination of a drug mixture in rats. Behav Pharmacol. 1996;7:364–372. doi: 10.1097/00008877-199608000-00008. [DOI] [PubMed] [Google Scholar]
- McClure RK. The visual backward masking deficit in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:301–311. doi: 10.1016/s0278-5846(00)00166-4. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carrera MRA, Fratta W, Valverde O, Cossu G, Fattore L, Chowen JA, Gómez R, del Arco I, Villanua, Maldonado R, Koob GF, de Fonseca FR. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK. Effects of mu opioid agonists alone and in combination with cocaine and D-amphetamine in rhesus monkeys trained to discriminate cocaine. Neuropsychopharmacology. 1998;18:325–338. doi: 10.1016/S0893-133X(97)00163-2. [DOI] [PubMed] [Google Scholar]
- Nencini P, Woolverton WL. Effects of nimodipine on the discriminative stimulus properties of d-amphetamine in rats. Psychopharmacology. 1988;96:40–44. doi: 10.1007/BF02431531. [DOI] [PubMed] [Google Scholar]
- Overton DA. Test for a neurochemically specific mechanism mediating drug discriminations and for stimulus masking. Psychopharmacology. 1983;81:340–344. doi: 10.1007/BF00427574. [DOI] [PubMed] [Google Scholar]
- Platt DM, Grech DM, Rowlett JK, Spealman RD. Discriminative stimulus effects of morphine in squirrel monkeys: stimulants, opioids, and stimulant-opioid combinations. J Pharmacol Exp Ther. 1999;290:1092–1100. [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. Mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Involvement of mu-, delta- and kappa-opioid receptor subtypes in the discriminative-stimulus effects of delta-9-tetrahydrocannabinol (THC) in rats. Psychopharmacology (Berl) 2005;179:804–812. doi: 10.1007/s00213-004-2118-x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Antoniou K, Pappas LA, Goldberg SR. The cannabinoid CB1 antagonist N-piperidinyl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl) -4-methylpyrazole-3-carboxamide (SR-141716A) differentially alters the reinforcing effects of heroin under continuous reinforcement, fixed ratio, and progressive ratio schedules of drug self-administration in rats. J Pharmacol Exp Ther. 2003;306:93–102. doi: 10.1124/jpet.102.047928. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- Solinas M, Zangen A, Thiriet N, Goldberg SR. Beta-endorphin elevations in the ventral tegmental area regulate the discriminative effects of Delta-9-tetrahydrocannabinol. Eur J Neurosci. 2004;19:3183–3192. doi: 10.1111/j.0953-816X.2004.03420.x. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mariathasan EA, White JA. Influencing the specificity of drug mixture discriminations by varying the training procedure. Behav Pharmacol. 1999;10:657–664. doi: 10.1097/00008877-199911000-00012. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, White JA. Impact of training history on discrimination of a drug mixture by rats. Behav Pharmacol. 1996;7:483–494. [PubMed] [Google Scholar]
- Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms--a review of recent preclinical data. Psychopharmacology (Berl) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, de Wit H. Naltrexone does not block the subjective effects of oral Delta(9)-tetrahydrocannabinol in humans. Drug Alcohol Depend. 2000;59:251–260. doi: 10.1016/s0376-8716(99)00127-1. [DOI] [PubMed] [Google Scholar]
- Wegel RL, Lane CE. The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear. Physiol Rev. 1924;23:266–285. [Google Scholar]
- Welch SP, Eads M. Synergistic interactions of endogenous opioids and cannabinoid systems. Brain Res. 1999;848:183–190. doi: 10.1016/s0006-8993(99)01908-3. [DOI] [PubMed] [Google Scholar]
- Wiley JL. Cannabis: discrimination of “internal bliss”? Pharmacol Biochem Behav. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Young AM, Masaki MA, Geula C. Discriminative stimulus effects of morphine: effects of training dose on agonist and antagonist effects of mu opioids. J Pharmacol Exp Ther. 1992;261:246–257. [PubMed] [Google Scholar]