Table 1. Binding of urea compounds to T. brucei MetRS by a thermal shift assay.
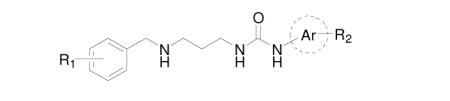
| Compound | Ri | Aromatic ring |
R2 |
T. brucei MetRS ΔTm (°C)a |
|---|---|---|---|---|
| 2 | 3,5-diCl | phenyl | H | 7.8±0.3 |
| 4 | 3-Cl,5-OMe | phenyl | H | 7.7±0.1 |
| 5 | 2,3,5-TriCl | phenyl | H | 6.4±0.3 |
| 6 | 3-Cl | phenyl | H | 5.5±0.1 |
| 7 | 3-OMe | phenyl | H | 4.7±0.1 |
| 8 | 3-OEt | phenyl | H | 2.7±i.3 |
| 9 | 4-Cl | phenyl | H | 2.5±0.i |
| 10 | 2-Cl | phenyl | H | 1.9±0.2 |
| 11 | 4-OMe | phenyl | H | 1.1±0.3 |
| 12 | 2-OEt | phenyl | H | 1.1±0.3 |
| 13 | H | phenyl | H | 1.3±0.1 |
| 14 | 2-OMe | phenyl | H | 1.1±0.1 |
| 15 | 4-OEt | phenyl | H | 0.5±0.2 |
|
| ||||
| 16 | 3,5-diCl | Phenyl | 2-OH | 8.4±0.3 |
| 17 | 3,5-diCl | Phenyl | 3-OH | 7.7±0.i |
| 18 | 3,5-diCl | Phenyl | 3-F | 7.7±0.i |
| 19 | 3,5-diCl | phenyl | 2-F | 7.3±0.i |
| 20 | 3,5-diCl | Phenyl | 4-F | 6.3±0.5 |
| 21 | 3,5-diCl | Phenyl | 4-Cl | 5.7±0.2 |
| 22 | 3,5-diCl | Phenyl | 3-Cl | 5.2±0.3 |
| 23 | 3,5-diCl | Phenyl | 4-OH | 5.8±0.3 |
| 24 | 3,5-diCl | Phenyl | 2-NH2 | 4.7±0.2 |
| 25 | 3,5-diCl | phenyl | 3-NH2 | 4.3±0.2 |
|
| ||||
| 26 | 3,5-diCl | 3-thiophene | H | 8.3±0.9 |
| 27 | 3-Cl,5-OMe | 3-thiophene | H | 7.9±0.i |
| 28 | 3,5-diCl | 2-thiophene | H | 7.6±0.3 |
| 29 | 3,5-diCl | 3-pyridine | H | 7.1±0.1 |
| 30 | 3,5-diCl | 2-pyridine | H | 4.5±0.2 |
| 31 | 3,5-diCl | 4-pyridine | H | 3.1±0.1 |
ΔTm values are the average of three independent runs.
