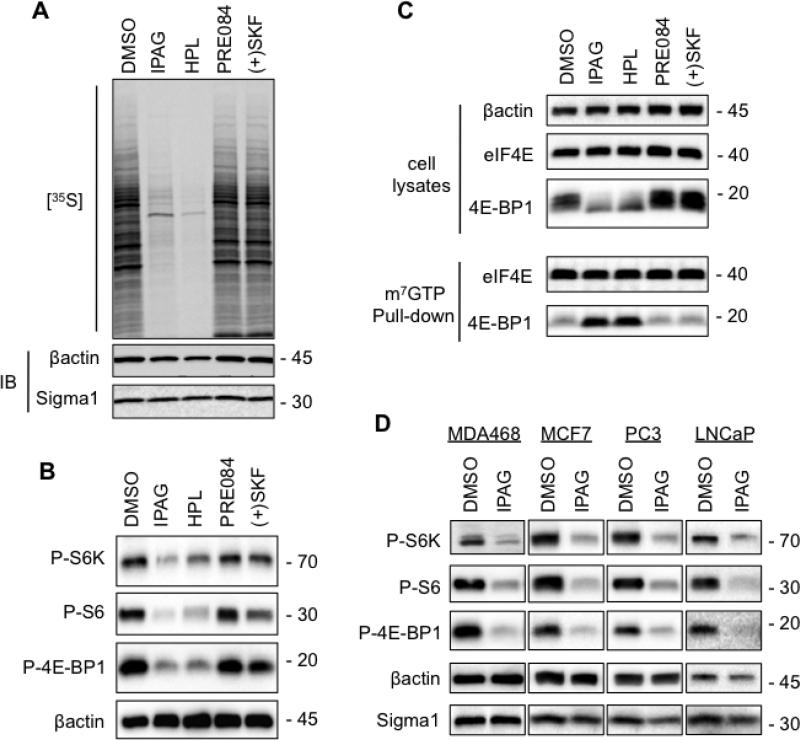
Fig. 2. Inhibition of cap-dependent translation initiation mediated by Sigma1 antagonist.
(A.) T47D cells were treated for 24 hours with 10μM antagonists (IPAG, haloperidol) or 10μM agonists (PRE084, (+)SKF-10047). Subsequently, cells were pulse-labeled for 1 hour with 100μCi/ml [35S] methionine and cysteine. Protein extracts were resolved by SDS-PAGE, transferred onto nitrocellulose membrane and exposed to autoradiography film. (B.) Immunoblots of protein extracts from T47D cells treated for 24 hours with 10μM putative antagonists (IPAG, haloperidol) or putative agonists (PRE084, (+)SKF-10047). Phospho-threonine 389-p70S6K (P-S6K), phospho-serine 235/236 S6 (P-S6), and phospho-serine 65-4E-BP1 (P-4E-BP1). (C.) Cell lysates were precipitated with m7GTP-sepharose beads (pull-down) and subsequently immunoblotted with antibodies against 4E-BP1 and eIF4E to evaluate 4E-BP1 binding to the eIF4E-mRNA cap complex. The upper panel (cell lysates) demonstrates equivalent input into m7GTP-sepharose binding, as well as Sigma1 ligand mediated changes in 4E-BP1 phosphorylation profile. (D.) Immunoblots of protein extracts from breast (MDA468, MCF7) and prostate adenocarcinoma (PC3, LNCaP) cells treated for 24 hours with 10μM IPAG.