Abstract
Survivors of pediatric medulloblastoma are at risk for neurocognitive dysfunction. Reduced white matter integrity has been correlated with lower intelligence in child survivors, yet associations between specific cognitive processes and white matter have not been examined in long-term adult survivors. Twenty adult survivors of medulloblastoma were randomly recruited from a larger institutional cohort of adult survivors of childhood cancer. Survivors underwent comprehensive neurocognitive evaluations and MRI. Data on brain volume and cortical thickness and diffusion tensor imaging were acquired, including measures of fractional anisotropy, apparent diffusion coefficient, and axial and radial diffusivity. Observed neurocognitive scores were compared with population norms and correlated to MRI indices. Survivors were, on average, 29 years of age and 18 years postdiagnosis. Mean full-scale intelligence quotient was nearly 1 SD below the normative mean (86.3 vs 100, P = .004). Seventy-five percent of survivors were impaired on at least one measure of executive function. Radial diffusivity in the frontal lobe of both hemispheres was correlated with shifting attention (left: rs = −0.67, P = .001; right: rs = −0.64, P = .002) and cognitive flexibility (left: rs = −0.56, P = .01; right: rs = −0.54, P = .01). Volume and cortical thickness were not correlated with neurocognitive function. Neurocognitive impairment was common and involved many domains. Reduced white matter integrity in multiple brain regions correlated with poorer performance on tasks of executive function. Future research integrating diffusion tensor imaging should be a priority to more rigorously evaluate long-term consequences of cancer treatment and to inform cognitive intervention trials in this high-risk population.
Keywords: diffusion tensor imaging, executive function, medulloblastoma, neurocognition
Malignancies of the central nervous system have an incidence of approximately 3.0 per 100 000 persons ≤19 years of age in the United States.1 Primitive neuroectodermal tumors, which include medulloblastoma (MB), account for 10%–20% of all pediatric brain tumors and 40% of posterior fossa tumors.2,3 While over 80% of children diagnosed with standard-risk MB achieve long-term survival,4 the consequences of tumor location within the cerebellum and treatment with craniospinal irradiation (CSI) are considerable. In this paper, we review recent literature on neurocognitive outcomes and brain integrity in survivors of MB and present pilot data from adult survivors of childhood MB who are now 12–25 years postdiagnosis and post–initial treatment.
Neurocognitive deficits are among the most common sequelae observed in CSI treatment for MB. Deficits in intelligence, academics, attention, processing speed, memory, and executive function are well documented shortly following therapy completion.5–7 Younger age at diagnosis (ie, < 7 years of age), higher dose CSI compared to reduced-dose CSI, female sex, and clinical complications such as hydrocephalus and posterior fossa syndrome have been associated with poorer cognitive outcomes, although CSI dose is most consistently implicated in neurocognitive dysfunction.8–10 Detrimental effects of CSI on cognition at dose levels ≥36 Gy compared with doses ≤23.4 Gy have been reported,11 yet these effects appear to be moderated by age at diagnosis.5
Few studies have investigated long-term neurocognitive functioning in adult survivors of childhood CNS malignancies. Edelstein and colleagues12 reported performance-based neurocognitive outcomes for 20 adult survivors of childhood MB aged 18–47 years who were 6–42 years postdiagnosis. Compared with normative data, the survivors demonstrated global cognitive deficits and performed 1.2 SD below the expected mean in the area of working memory, 2.4 SD below in processing speed, and 3.4 SD below in executive function. Using the Childhood Cancer Survivor Study (CCSS), Ellenberg and colleagues13 reported on the neurocognitive status in over 800 adult survivors of childhood CNS tumors. Compared with non–CNS tumor survivors and siblings, CNS tumor survivors were significantly more likely to self-report impairment in the areas of task efficiency, memory, organization, and emotional regulation. Survivors treated with whole brain cranial radiation were significantly more likely to report problems with task efficiency (effect size = 0.65) and memory (effect size = 0.63) compared with CNS tumor survivors who did not receive cranial radiation. In a follow-up CCSS investigation, Armstrong and colleagues14 reported that region-specific cranial radiation to the temporal lobe was associated with increased likelihood of impaired task efficiency and organization in adult survivors of MB.
Cranial irradiation–induced cerebral white matter injury has been postulated to contribute to neurocognitive dysfunction in MB survivors. Mulhern et al15 reported significantly less normal-appearing white matter and lower full-scale intelligence quotient (FSIQ) in 18 MB patients treated with CSI compared with age-matched patients treated for low-grade posterior fossa tumors with surgery alone. In MB patients, white matter volume was positively correlated with FSIQ. In a longitudinal study of 26 cases of MB treated with risk-adapted CSI, patients demonstrated significant loss of white matter relative to normally expected maturation.16 No significant difference in rate of volume loss was observed by age at CSI treatment; however, patients treated with 23.4 Gy CSI demonstrated lower rate of volume loss compared with patients treated with 36 Gy CSI. Palmer et al17 showed longitudinal white matter volume loss in posterior regions of the corpus callosum in 35 cases of MB treated with risk-adapted CSI; however, decline in volume was not statistically different among patients who were treated with conventional compared with reduced-dose CSI. In the first study to employ age-similar healthy controls, Reddick et al18 reported that MB survivors treated with 35–40 Gy CSI demonstrated significantly less development of normal-appearing white matter and that younger age at CSI treatment and ventricular shunt placement were associated with reduced white matter volume in survivors.
Importantly, white matter volume loss has been associated with neurocognitive deficits in MB survivors treated with CSI. In a study of 42 survivors, 1–11 years post-treatment, Mulhern et al19 found that white matter volume accounted for a significant amount of the variance in the relationship of age at CSI and intellectual functioning, including verbal and nonverbal reasoning, but white matter volume was not significantly associated with performance on tasks of sustained attention or verbal memory. Data from a heterogeneous sample20 of 40 brain tumor survivors, including 18 MB patients, also revealed an association between decreased white matter volume and neurocognitive deficits, specifically global intelligence and attention. Importantly, this study further suggested that attentional abilities may mediate the observed relationship between white matter volume and IQ.
While volume loss provides an important metric for quantifying white matter injury, it does not provide insight into microscopic changes that may affect integrity of existing white matter. MRI with diffusion tensor imaging (DTI) has emerged as a useful technique to quantify water diffusion within white matter tracts. Fractional anisotropy (FA) provides an index of directionality of diffusion due to parallel organization of axonal fibers, with greater FA reflecting a higher degree of myelination and density, or white matter integrity. Diffusivity refers to the magnitude of diffusion of water molecules, which is restricted perpendicular to axonal fibers and can be determined by measuring the apparent diffusion coefficient (ADC) and its components of axial diffusivity (AX) and radial diffusivity (RAD), with greater diffusivity reflecting myelin-specific abnormalities. Despite the high sensitivity of this technique to pathological and functional changes within cerebral white matter, few studies have applied this method to study white matter damage in relation to neurocognitive function in survivors of MB.
Khong et al21 utilized DTI to examine white matter integrity in 9 MB patients 1–6 years posttreatment and age-matched controls. MB survivors evidenced reduced FA in cerebral hemispheres, pons, medulla, frontal and parietal periventricular white matter, and corona radiata compared with controls. In a subsequent study of 20 MB survivors treated with CSI and boosts to the posterior fossa who were 0.2–5.8 years post-CSI, greater percentage change in FA (ie, greater loss of white matter anisotropy) was associated with younger age at diagnosis and higher CSI dose.22 Khong et al23 further reported an association between white matter anisotropy and neurocognitive function in child survivors of MB and acute lymphoblastic leukemia (ALL). Loss of FA was measured in 12 MB patients treated with CSI, 9 ALL patients treated with cranial radiation, and 9 ALL patients treated with chemotherapy alone. Percentage FA difference between survivors and age-matched controls was significantly associated with FSIQ as well as verbal and nonverbal reasoning.
Mabbott et al24 used DTI to investigate white matter integrity following CSI treatment in 8 cases of MB. Relative to age-matched controls, MB patients had lower FA in several regions of interest, including the genu of the corpus callosum, posterior and anterior limbs of the internal capsule, and inferior and superior frontal white matter. The ADC in survivors was higher in these same regions, as well as in the parietal white matter. In survivors, reduced global intelligence was correlated with decreased FA. Palmer et al25 examined the relationship between reading skills and white matter integrity in 54 MB patients 12 months postdiagnosis. After adjusting for patient age and treatment risk status, reading decoding skills were significantly positively associated with FA in the left pons-medulla, right pons, left and right posterior limb of the internal capsule, right knee of the internal capsule, left inferior parietal, right occipital lobe, and left temporal occipital cluster.
To date, only one study has examined DTI in MB survivors in relation to more specific cognitive processes thought to underlie global intelligence. Aukema et al26 examined white matter FA and speed of processing in childhood cancer survivors aged 8–16 years. Patients included 6 MB survivors, 5 ALL survivors treated with high-dose methotrexate, and 6 ALL survivors treated with low-dose methotrexate. Compared with age-matched controls, white matter FA was reduced in the right inferior fronto-occipital fasciculus (IFO) and the genu of the corpus callosum. White matter FA in the splenium and body of the corpus callosum was significantly positively correlated with processing speed, while white matter FA in the right IFO was positively correlated with motor speed.
DTI has been used to investigate susceptibility of specific brain regions to radiation-induced injury. Qiu et al27 compared white matter FA in the frontal and parietal lobes of 22 MB survivors treated with equivalent doses of whole-brain radiation with age- and gender-matched controls. White matter FA was reduced in both regions in MB survivors compared with controls. Among survivors, frontal lobe white matter FA was more severely reduced than parietal lobe white matter, suggesting increased white matter sensitivity in frontal lobes. These data suggest that cognitive processes mediated by the frontal lobe, such as executive functioning, may be differentially affected following treatment-induced white matter injury. Recently, Rueckriegel et al28 investigated supratentorial white matter damage in survivors of posterior fossa tumors, including 17 cases of MB treated with CSI and 13 cases of pilocytic astrocytoma treated with surgery alone, as well as age-matched controls. Compared with controls, MB survivors showed reduced FA in cerebellar midline structures, frontal lobes, and callosal body. No differences were apparent when comparing cases of MB with those of pilocytic astrocytoma for specific regions of interest; however, the amount of significantly reduced FA was greater in MB survivors.
Despite growing evidence implicating white matter injury as a contributing factor to the cognitive dysfunction experienced by MB survivors, no study has examined the association between white matter integrity following CSI treatment and cognition in long-term adult survivors of childhood MB. We report pilot data from such a study below.
Methods
Patients
Twenty MB survivors registered in the St Jude Lifetime Cohort Study29 were randomly recruited. Eligibility criteria for the sample included a diagnosis of MB treated at St Jude Children's Research Hospital, tumor location within the posterior fossa, treatment with CSI, current age ≥18 years, and ≥10 years postdiagnosis. Exclusion criteria included history of developmental disorder or neurological event unrelated to cancer. All survivors provided informed written consent, and the study protocol was approved by the St Jude Children's Research Hospital Institutional Review Board.
Procedure
Medical record abstraction was performed for radiation treatment (fields, doses, and energy source), all chemotherapy received (cumulative doses), and surgical procedures. Survivors underwent comprehensive assessment for neurocognitive functions in dedicated evaluation rooms. Assessed domains included intelligence (Wechsler Abbreviated Scale of Intelligence),30 academic skills (Woodcock–Johnson Tests of Achievement III),31 attention (Trail Making Test, Part A; Connors Continuous Performance Test II–Variability),32,33 memory (Wechsler Adult Intelligence Scale [WAIS] III–Digits Forward; California Verbal Learning Test II; Recognition Memory Test),34–36 processing speed (WAIS III–Digit Symbol Coding and Symbol Search; Grooved Pegboard Test),32,34 motor function (Finger Tapping Test; Hand Dynamometer),37 and executive function (Wisconsin Card Sorting Test; Rey–Osterrieth Complex Figure; Stroop Color Word; Trail Making Test, Part B; WAIS III–Digits Backward; Controlled Oral Word Association Test).32,34,38–41 Survivors also completed self-ratings to assess behavioral and cognitive symptoms of executive dysfunction (Behavior Rating Inventory of Executive Function).42 Order of testing was standardized and all assessments were completed under the supervision of a licensed psychologist. The interval between neurocognitive testing and MRI was 0–9 days.
Magnetic Resonance Imaging
MRI was acquired on one of two 1.5-Tesla Avanto MR scanners (Siemens Medical Systems) using bipolar diffusion-encoding gradients. All images were acquired using a double spin echo planar imaging pulse sequence (repetition time/echo time = 10/100 ms, b = 1000 ms). Imaging sets were acquired as forty 3-mm-thick contiguous axial sections with whole-head coverage, 128 square matrix, and 22-cm field of view (acquired resolution of 1.7 × 1.7 × 3.0 mm). Four acquisitions were made with 12 noncollinear, noncoplanar diffusion gradient directions to calculate the diffusion tensor for each voxel.
Voxel-wise tensor calculations were performed with the DTI toolkit under SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Data from the 4 acquisitions were realigned before tensor calculation to correct for linear image drift. FA, ADC, AX, and RAD maps were calculated for the whole brain. Parametric maps were registered to the International Consortium for Brain Mapping average 152 T2 atlas aligned in Talairach space and resampled to a 1-mm isotropic resolution. Once registered, average values were calculated for each of the parameters from white matter within each of the 6 lobular regions (left and right frontal, parietal, and temporal).
MRIs were also processed with FreeSurfer software (http://surfer.nmr.mgh.harvard.edu/) to assess brain cortical thickness. Images were aligned to correct radiofrequency inhomogeneities, brain extraction was conducted, and segmentation was used to create pial and gray/white matter surfaces to calculate the perpendicular distance or thickness. Automated parcellation of the brain was conducted to extract thickness for specific frontal, parietal, temporal, and occipital regions.
Statistical Analyses
Descriptive statistics were calculated for demographic and treatment characteristics as well as neurocognitive outcomes. Scores on neurocognitive measures were transformed into age-adjusted z-scores using national normative data (M = 0, SD = 1). Quantile-quantile plots and the Shapiro–Wilk test were used to assess normality. To account for nonnormally distributed data, nonparametric statistical tests were employed for all analyses. Mean neurocognitive scores for the sample were compared with population norms using the one-sample Wilcoxon signed rank test. Impairment was defined as a score ≥1.3 SD below the expected population mean, corresponding to a performance consistent with the lowest 10th percentile of normative data. MRI measures were correlated with performance on executive function tasks and age at diagnosis using Spearman's rank correlation. To account for multiple comparisons, only correlations at P ≤ .01 were considered statistically significant. All statistical analyses were completed in SAS version 9.2.
Results
The 20 MB survivors (14 male) were, on average, 29 years of age and 18 years postdiagnosis (Table 1). Age at diagnosis ranged from 2 to 17 years. All patients were treated with >50 Gy total cumulative cranial irradiation, including a boost to the posterior fossa. Sixty percent of survivors were employed less than full time, and 50% were living dependently at the time of evaluation.
Table 1.
Patient characteristics
| Survivors (n = 20) |
||
|---|---|---|
| n | % | |
| Sex | ||
| Female | 6 | 30 |
| Male | 14 | 70 |
| Age at evaluation, y | ||
| 21–25 | 7 | 35 |
| 26–29 | 7 | 35 |
| 30–36 | 6 | 30 |
| Educational attainment | ||
| ≤High school | 6 | 30 |
| Training beyond HS/some college | 5 | 25 |
| ≥College graduate | 9 | 45 |
| Employment | ||
| Unemployed | 7 | 35 |
| Part-time | 5 | 25 |
| Full-time/student | 8 | 40 |
| Living situation | ||
| Independent | 10 | 50 |
| Dependent | 10 | 50 |
| Age at diagnosis, y | ||
| <7 | 4 | 20 |
| 7–10 | 7 | 35 |
| 11–14 | 6 | 30 |
| 15–17 | 3 | 15 |
| Time since diagnosis, y | ||
| 12–15 | 7 | 35 |
| 16–19 | 6 | 30 |
| 20–25 | 7 | 35 |
| Chemotherapy | ||
| Yes | 15 | 75 |
| No | 5 | 25 |
| Median | Range | |
| Radiation (cGy) | ||
| Cranium | 3520 | 2340–5500 |
| Spine | 3520 | 2340–6160 |
| Posterior fossa boost | 1800 | 1100–3240 |
Mean FSIQ was nearly 1 SD below the normative mean (86.3 vs 100, P = .004). Survivors demonstrated multiple areas of impairment, with median scores in all areas falling below the expected population mean on performance based measures (Table 2). Fifty-five percent of survivors were impaired in ≥2 areas of executive function. Age at diagnosis was correlated with FSIQ (rs = 0.70, P = .001), as well as cognitive fluency (rs = 0.60, P = .005), shifting attention (rs = 0.60, P = .006), working memory (rs = 0.77, P < .001), cognitive flexibility (rs = 0.61, P = .005), and planning and organization (rs = 0.57, P = .008). Table 3 provides a comparison of FSIQ and performance on measures of executive function by median age at diagnosis.
Table 2.
Neurocognitive functioning
| Median | IQ Range | % Impairmenta | P-valueb | |
|---|---|---|---|---|
| Intelligence | ||||
| Full scale | −0.90 | −2.0, 0.00 | 35 | 0.005 |
| Verbal | −1.0 | −2.2, 0.05 | 45 | 0.003 |
| Perceptual | −0.65 | −1.85, 0.1 | 30 | 0.02 |
| Academics | ||||
| Word reading | −0.50 | −1.8, −0.37 | 35 | <0.001 |
| Calculations | −1.33 | −2.7, −0.6 | 50 | <0.001 |
| Attention | ||||
| Focus | −1.23 | −4.73, −0.33 | 50 | <0.001 |
| Sustain | −0.20 | −1.1, 0.10 | 15 | 0.01 |
| Memory | ||||
| New learning | −1.1 | −1.9, −0.35 | 45 | <0.001 |
| Short-term | −1.0 | −1.5, −0.50 | 35 | <0.001 |
| Long-term | −1.5 | −2.0, −1.0 | 55 | <0.001 |
| Span | −1.23 | −2.0, 0.27 | 35 | 0.01 |
| Visual | −1.67 | −2.0, −1.0 | 70 | <0.001 |
| Processing speed | ||||
| Motor | −1.83 | −4.7, −1.16 | 75 | <0.001 |
| Information | −1.0 | −1.83, −0.33 | 45 | <0.001 |
| Visual-motor | −1.5 | −2.17, −0.83 | 60 | <0.001 |
| Motor | ||||
| Fine | −3.37 | −5.27, −2.08 | 80 | <0.001 |
| Gross | −2.20 | −2.63, −1.5 | 80 | <0.001 |
| Executive function | ||||
| Fluency | −0.33 | −1.17, 0.17 | 25 | 0.07 |
| Working memory | −0.67 | −1.27, −0.60 | 20 | <0.001 |
| Interference control | −0.4 | −0.5, −0.65 | 0 | 0.03 |
| Flexibility | −0.87 | −1.40, −0.43 | 35 | <0.001 |
| Shifting | −3.97 | −5.33, −0.87 | 65 | <0.001 |
| Planning and organization | −1.9 | −3.0, −0.7 | 55 | <0.001 |
| Behavior rating | ||||
| Inhibition | 0.65 | −0.15, 1.15 | 5 | 0.01 |
| Shift | 0.30 | −1.40, 0.70 | 25 | 0.88 |
| Emotional control | 0.70 | 0.2, 1.0 | 15 | 0.07 |
| Self-monitor | 0.60 | −0.45, 0.80 | 15 | 0.37 |
| Cognitive rating | ||||
| Initiation | 0.30 | −0.45, 0.60 | 10 | 0.78 |
| Working memory | −0.90 | −1.75, −0.30 | 35 | 0.005 |
| Planning | 0.25 | −0.85, 0.60 | 10 | 0.96 |
| Task completion | −0.20 | −0.90, 0.45 | 10 | 0.26 |
| Organization | −0.10 | −0.60, 0.50 | 20 | 0.57 |
aImpairment defined as performance ≤10th percentile.
bComparison of observed with expected mean (M = 0).
Table 3.
Executive function by age at diagnosis
| Diagnosis ≤10 y (n = 11) |
Diagnosis >10 y (n = 9) |
P-valuea | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| FSIQ | −1.70 | 0.92 | 0.04 | 0.84 | 0.002 |
| Fluency | −1.30 | 1.13 | 0.37 | 1.13 | 0.009 |
| Working memory | −1.52 | 1.02 | −0.34 | 0.47 | 0.001 |
| Shifting attention | −4.58 | 1.68 | −1.4 | 1.7 | 0.002 |
| Planning/organization | −2.65 | 1.23 | −0.88 | 0.99 | 0.005 |
| Flexibility | −1.42 | 0.73 | −0.29 | 0.58 | 0.004 |
a Mann–Whitney U-test for independent samples.
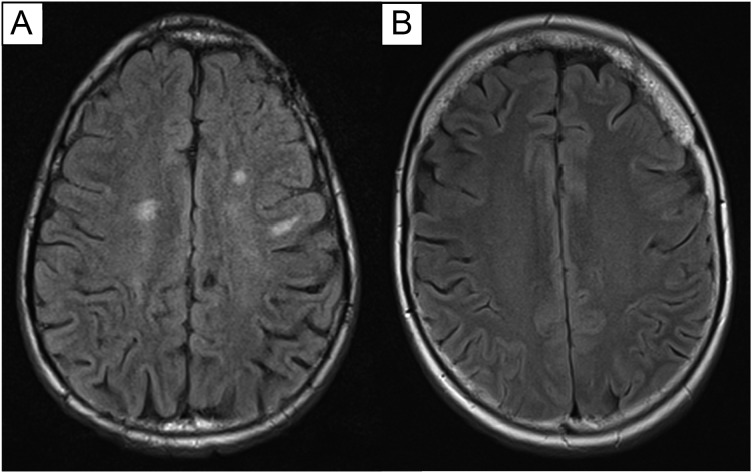
Sixteen of 20 survivors (80%) showed evidence of leukoencephalopathy on MRI. Leukoencephalopathy in the basal ganglia or brainstem was present in 6 survivors. Three of these 6 survivors had only subcortical leukoencephalopathy involvement, while 3 had both cortical and subcortical involvement. Thirteen survivors demonstrated leukoencephalopathy in the cortex, 5 of whom had only frontal involvement and 7 of whom had frontal and parietal/temporal involvement, including 2 with corona radiata involvement. One survivor had leukoencephalopathy involving only the corona radiata. Figure 1 provides an axial slice image for 2 survivors, with and without evidence of multifocal leukoencephalopathy. All survivors evidenced multifocal hemosiderin deposits, suggesting prior infarcts that were likely treatment related. Eight survivors also evidenced cavernous malformations, which accounted for some of the observed infarcts, but not all in any one patient.
Fig. 1.
Axial slices showing the presence of multifocal leukoencephalopathy in one survivor (A) and a survivor with no apparent white matter abnormalities (B).
White Matter Integrity and Executive Function
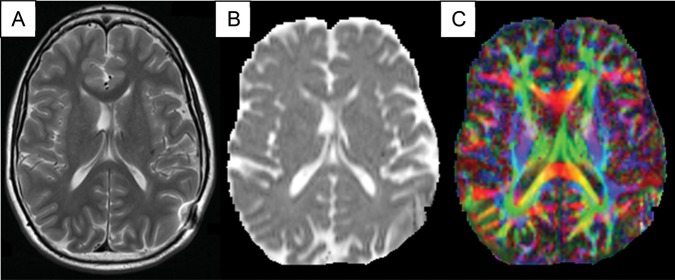
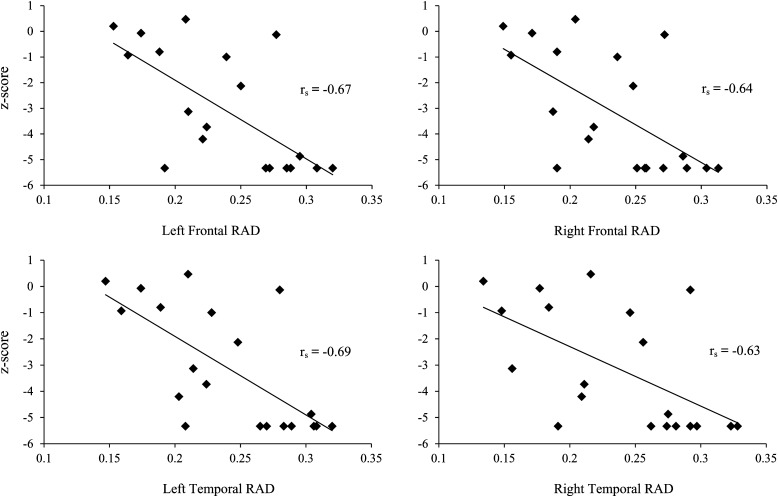
Figure 2 shows DTI images for a single patient. RAD in the frontal lobe of both hemispheres was negatively correlated with shifting attention (left: rs = −0.67, P = .001; right: rs = −0.64, P = .002) and cognitive flexibility (left: rs = −0.56, P = .01; right: r = −0.54, P = .01). The top panel of Fig. 3 shows the correlation between white matter RAD and shifting attention in the left and right hemispheres of the frontal lobe.
Fig. 2.
DTI for one MB patient. (A) T2-weighted image, axial slice. (B) Apparent diffusion coefficient map without directional information. (C) Fractional anisotropy directional map. Red: left-right; green: anteroposterior; blue: superior-inferior.
Fig. 3.
Correlation between shifting attention and radial diffusivity (RAD).
FA in the parietal lobe was positively correlated with working memory (right: rs = 0.52, P = .017; left: rs = 0.54, P = .01). RAD in both parietal lobes was negatively correlated with shifting attention (left: rs = −0.63, P = .003; right: rs = −0.55, P = .01).
In the temporal lobe, RAD was negatively correlated with shifting attention (right: rs = −0.63, P = .003; left: rs = −0.69, P = .0008) and cognitive flexibility (right: rs = −0.52, P = .017; left: rs = −0.56, P = .01). Cognitive fluency was positively correlated with FA in the left and right temporal lobes (left: rs = 0.65, P = .002; right: rs = 0.58, P = .007). The bottom panel of Fig. 3 shows the correlation between RAD and shifting attention in both hemispheres of the temporal lobe.
No statistically significant correlations were found between AX and measures of executive function for any brain region. Additionally, no significant correlations emerged between white matter volume or cortical thickness and performance on measures of executive function. Table 4 provides correlations between FA and RAD and other assessed domains of neurocognitive function for the 6 lobular regions described.
Table 4.
Correlations between measures of white matter integrity and neurocognitive functioning
| Fractional Anisotropy |
Radial Diffusivity |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left Frontal | Right Frontal | Left Temporal | Right Temporal | Left Parietal | Right Parietal | Left Frontal | Right Frontal | Left Temporal | Right Temporal | Left Parietal | Right Parietal | |
| Intelligence | ||||||||||||
| Full scale | 0.52 | 0.51 | 0.59a | 0.48 | 0.51 | 0.44 | −0.39 | −0.36 | −0.42 | −0.35 | −0.36 | −0.24 |
| Verbal | 0.42 | 0.40 | 0.50 | 0.44 | 0.44 | 0.42 | −0.40 | −0.37 | −0.44 | −0.39 | −0.36 | −0.27 |
| Perceptual | 0.48 | 0.49 | 0.54a | 0.44 | 0.48 | 0.43 | −0.30 | −0.29 | −0.31 | −0.27 | −0.28 | −0.18 |
| Academics | ||||||||||||
| Word reading | 0.35 | 0.37 | 0.46 | 0.45 | 0.42 | 0.49 | −0.44 | −0.42 | −0.46 | −0.43 | −0.41 | −0.34 |
| Calculations | 0.34 | 0.40 | 0.47 | 0.54a | 0.36 | 0.52a | −0.48 | −0.46 | −0.52 | −0.49 | −0.45 | −0.39 |
| Attention | ||||||||||||
| Focus | 0.28 | 0.31 | 0.43 | 0.41 | 0.26 | 0.34 | −0.41 | −0.39 | −0.44 | −0.40 | −0.37 | −0.30 |
| Sustain | −0.15 | −0.12 | −0.09 | 0.03 | −0.13 | −0.17 | −0.36 | −0.34 | −0.42 | −0.36 | −0.29 | −0.32 |
| Memory | ||||||||||||
| New learning | 0.37 | 0.31 | 0.27 | 0.23 | 0.33 | 0.35 | −0.25 | −0.22 | −0.22 | −0.22 | −0.24 | −0.19 |
| Short-term | 0.41 | 0.38 | 0.25 | 0.24 | 0.39 | 0.40 | −0.02 | −0.01 | 0.06 | 0.03 | −0.01 | 0.03 |
| Long-term | 0.00 | 0.03 | −0.09 | 0.11 | 0.08 | 0.35 | −0.07 | −0.11 | 0.03 | −0.10 | −0.11 | −0.13 |
| Span | 0.52 | 0.45 | 0.53 | 0.28 | 0.66a | 0.34 | −0.26 | −0.17 | −0.31 | −0.18 | −0.25 | −0.10 |
| Visual | 0.50 | 0.59a | 0.57a | 0.66a | 0.58 | 0.78b | −0.19 | −0.15 | −0.17 | −0.02 | −0.19 | 0.13 |
| Processing speed | ||||||||||||
| Information | 0.52 | 0.53 | 0.68a | 0.56a | 0.53 | 0.49 | −0.29 | −0.25 | −0.35 | −0.27 | −0.26 | −0.16 |
| Visual-motor | 0.44 | 0.47 | 0.61a | 0.57a | 0.47 | 0.45 | −0.28 | −0.26 | −0.34 | −0.27 | −0.24 | −0.16 |
| Motor | 0.36 | 0.41 | 0.54a | 0.52 | 0.35 | 0.32 | −0.30 | −0.30 | −0.34 | −0.30 | −0.25 | −0.19 |
| Executive function | ||||||||||||
| Fluency | 0.46 | 0.44 | 0.65a | 0.58a | 0.51 | 0.44 | −0.44 | −0.39 | −0.53a | −0.44 | −0.38 | −0.28 |
| Working memory | 0.31 | 0.27 | 0.38 | 0.35 | 0.54a | 0.52a | −0.22 | −0.23 | −0.23 | −0.27 | −0.24 | −0.19 |
| Interference control | −0.24 | −0.42 | −0.21 | −0.36 | −0.09 | −0.19 | −0.28 | −0.28 | −0.27 | −0.25 | −0.30 | −0.26 |
| Flexibility | 0.23 | 0.24 | 0.17 | 0.19 | 0.29 | 0.22 | −0.56a | −0.54a | −0.56a | −0.52a | −0.51 | −0.44 |
| Shifting | 0.26 | 0.29 | 0.46 | 0.51 | 0.34 | 0.44 | −0.67b | −0.64a | −0.69b | −0.63a | −0.63a | −0.55a |
| Planning and organization | 0.34 | 0.39 | 0.25 | 0.46 | 0.36 | 0.46 | −0.32 | −0.31 | −0.27 | −0.27 | −0.28 | −0.25 |
| Behavior rating | ||||||||||||
| Inhibition | 0.10 | 0.22 | 0.06 | 0.34 | 0.10 | 0.35 | 0.33 | 0.29 | 0.39 | 0.32 | 0.36 | 0.27 |
| Shift | 0.35 | 0.46 | 0.28 | 0.15 | 0.13 | 0.28 | 0.41 | 0.37 | 0.34 | 0.30 | 0.47 | 0.38 |
| Emotional control | 0.23 | 0.21 | 0.15 | 0.25 | 0.19 | 0.26 | 0.21 | 0.21 | 0.26 | 0.27 | 0.27 | 0.18 |
| Self-monitor | 0.29 | 0.38 | 0.21 | 0.48 | 0.16 | 0.40 | 0.33 | 0.30 | 0.33 | 0.25 | 0.40 | 0.30 |
| Cognitive rating | ||||||||||||
| Initiation | 0.24 | 0.31 | 0.24 | 0.53 | 0.17 | 0.37 | 0.17 | 0.13 | 0.10 | 0.08 | 0.27 | 0.15 |
| Working memory | 0.42 | 0.48 | 0.36 | 0.56 | 0.27 | 0.27 | 0.19 | 0.18 | 0.15 | 0.19 | 0.27 | 0.24 |
| Planning | 0.21 | 0.30 | 0.20 | 0.50 | 0.23 | 0.47 | 0.18 | 0.14 | 0.17 | 0.10 | 0.22 | 0.14 |
| Task completion | 0.29 | 0.28 | 0.15 | 0.35 | 0.16 | 0.24 | 0.37 | 0.35 | 0.40 | 0.31 | 0.41 | 0.34 |
| Organization | 0.39 | 0.48 | 0.25 | 0.43 | 0.17 | 0.33 | 0.30 | 0.27 | 0.32 | 0.26 | 0.34 | 0.32 |
a P ≤ .01.
b P ≤ .001.
Discussion
The results of this pilot study of survivors of childhood MB treated with CSI revealed global neurocognitive impairment nearly 20 years postdiagnosis. Reduced white matter integrity was associated with observed neurocognitive dysfunction in these survivors. While past studies have reported correlations between general intelligence and white matter integrity in childhood survivors, our results suggest a relationship between specific executive function and white matter integrity in adult survivors treated with high-dose CSI for childhood MB.
Executive functions are higher-order cognitive processes that include shifting attention, working memory, cognitive fluency, cognitive flexibility, and planning and organization. The fronto-parietal network involves the dorsolateral prefrontal cortex, the anterior cingulate cortex, and the inferior and superior parietal lobes and has been implicated in supporting the integration and control of executive processes.43 While we did not examine white matter integrity within specific tracts known to contribute to this network, we found several significant correlations between white matter in the frontal and parietal lobes and executive processes. Specifically, white matter RAD in the frontal lobes was negatively correlated with performance on tasks of shifting attention (set shifting) and cognitive flexibility, while FA was positively correlated with working memory in the parietal lobe. Our findings suggest that loss of white matter integrity observed following CSI treatment persists for several decades and may further be associated with long-term deficits in executive function.
We also found associations between reduced white matter integrity within the temporal lobe and executive dysfunction, specifically cognitive flexibility, shifting attention, and cognitive fluency. Moreover, the observed associations between white matter integrity and executive function were generally not lateralized to a specific hemisphere. This suggests that integrated white matter pathways across several cortical regions may be responsible for the control of higher-order executive processes in MB survivors. Notably, we did not find significant associations between self-report of executive function and DTI measures. In fact, mean scores on measures of self-reported cognitive and behavior ratings did not differ from normative expectations, with the exception of working memory and inhibition. These data may suggest that performance-based measures may be more sensitive to subtle neurocognitive processes and underlying neuroanatomical changes. Alternatively, it may be that survivors adapt to their cognitive deficits over time, and self-report methods may potentiate response shift effects, whereby survivors' reporting reflects their recalibrated perceptions of cognitive function over time.
The most significant executive function deficit was observed on a task of cognitive set shifting, and poorer performance on this task was consistently correlated with reduced white matter integrity across several brain regions. Performance on executive function tasks are often multidetermined and may involve both executive and nonexecutive function components. The measure of cognitive set shifting employed in this study also required visual-motor demands (Trail Making Test, Part B). However, performance on a cognitive task requiring comparable visual scanning and motor demands (Trail Making Test, Part A) was not significantly associated with any measures of white matter integrity. Thus, our results suggest that the specific ability to shift cognitive sets is associated with reduced white matter integrity, independent of visual scanning and motor demands.
Importantly, variability in neurocognitive outcomes was evident, even in our small sample of survivors. While a greater proportion of survivors were impaired relative to normative expectations, a sizable percentage of survivors (25%) did not evidence impairment on tasks of executive function. This finding demonstrates the need for genetic studies to understand variability in neurocognitive outcomes and/or sensitivity to CSI treatment–induced white matter damage. To date, studies have focused on polymorphisms believed to affect antioxidant enzyme activity. A study of child and adolescent MB patients demonstrated an association between glutathione s-transferase (GST)M1 null genotype and significant declines in global intelligence compared with GSTM1 nonnull genotypes following CSI.44 Recently, Brackett et al45 also reported associations between the GSTM1 polymorphism and greater psychological distress in adult survivors of childhood MB, though no significant associations between genotypes and self-report of neurocognitive function emerged. How genetic polymorphisms may be related to susceptibility to white matter damage following cranial irradiation in cases of MB is unknown.
A current endeavor within the field of pediatric oncology includes efforts toward remediation and/or rehabilitation of cognitive deficits that may emerge following neurotoxic cancer treatments. Several studies provide preliminary support for the effects of rehabilitation of neurocognitive deficits in childhood cancer survivors,46–48 though only one study has examined functional brain changes as a result of systematic training efforts.49 Thus, the neuronal mechanisms underlying the effects of cognitive intervention are largely unexplored and provide a promising area for future research. Evidence of white matter changes following cognitive intervention exists in noncancer populations, suggesting increased FA and decreased diffusivity post-intervention.50,51 It will be important to investigate the presence of potential connectivity changes in patients with known risk for white matter abnormalities following targeted intervention efforts.
While our data, considered in the context of past findings, suggest the need for future research, there are notable limitations. First, in the absence of an age-matched control group, we are unable to discuss how observed correlations between executive function and white matter integrity may be similar or dissimilar in healthy adults of the same age. Given the small sample size in this pilot study, we had limited power to detect statistically significant associations. However, the magnitude of observed correlations is considered large for behavioral research. Finally, as the patients in our sample were treated over a decade ago, the observed white matter and neuropsychological morbidity is likely greater than that experienced in cases of MB treated on more contemporary protocols, which often involve reduced CSI dose and advanced radiation technology (eg, conformal, proton beam).
In summary, in our sample of adult survivors of childhood MB, neurocognitive impairment was common and apparent across many specific domains of function. Reduced white matter integrity, as measured by FA and RAD, was associated with poorer performance on tasks of executive function. We suggest that future studies of neurotoxicity among MB survivors integrate DTI while also considering potential genetic predictors, to identify exposure-specific risks for adverse outcomes. Lastly, priority should be given to the design and testing of innovative cognitive interventions among this high-risk population.
Funding
This work was supported in part by the American Lebanese-Syrian Associated Charities (ALSAC) at St Jude Children's Research Hospital.
Conflict of interest. None declared.
References
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. 2011 Available at http://seer.cancer.gov/csr/1975_2008/ . Accessed April 10, 2012. [Google Scholar]
- 2.Partap S, Curran EK, Propp JM, Le GM, Sainani KL, Fisher PG. Medulloblastoma incidence has not changed over time: a CBTRUS study. J Pediatr Hematol Oncol. 2009;31:970–971. doi: 10.1097/MPH.0b013e3181bbc502. doi:10.1097/MPH.0b013e3181bbc502. [DOI] [PubMed] [Google Scholar]
- 3.CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008 (March 23, 2012 Revision) 2012 Available at http://www.cbtrus.org/ . Accessed April 10, 2012. [Google Scholar]
- 4.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma–96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. doi:10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 5.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. doi:10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 6.Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22:159–168. doi: 10.1037/0894-4105.22.2.159. doi:10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- 7.Jain N, Krull KR, Brouwers P, Chintagumpala MM, Woo SY. Neuropsychological outcome following intensity-modulated radiation therapy for pediatric medulloblastoma. Pediatr Blood Cancer. 2008;51:275–279. doi: 10.1002/pbc.21580. doi:10.1002/pbc.21580. [DOI] [PubMed] [Google Scholar]
- 8.Kieffer-Renaux V, Bulteau C, Grill J, Kalifa C, Viguier D, Jambaque I. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospatial irradiation doses. Dev Med Child Neurol. 2000;42:741–745. doi: 10.1017/s0012162200001377. doi:10.1017/S0012162200001377. [DOI] [PubMed] [Google Scholar]
- 9.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22:706–713. doi: 10.1200/JCO.2004.05.186. doi:10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 10.Grill J, Renaux VK, Bulteau C, et al. Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys. 1999;45:137–145. doi: 10.1016/s0360-3016(99)00177-7. doi:10.1016/S0360-3016(99)00177-7. [DOI] [PubMed] [Google Scholar]
- 11.Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- 12.Edelstein K, Spiegler BJ, Fung S, et al. Early aging in adult survivors of childhood medulloblastoma: long-term neurocognitive, functional, and physical outcomes. Neuro Oncol. 2011;13:536–545. doi: 10.1093/neuonc/nor015. doi:10.1093/neuonc/nor015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellenberg L, Liu Q, Gioia G, et al. Neurocognitive status in long-term survivors of childhood CNS malignancies: a report from the Childhood Cancer Survivor Study. Neuropsychology. 2009;23:705–717. doi: 10.1037/a0016674. doi:10.1037/a0016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;12:1173–1186. doi: 10.1093/neuonc/noq104. doi:10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulhern RK, Reddick WE, Palmer SL, et al. Neurocognitive deficits in medulloblastoma survivors and white matter loss. Ann Neurol. 1999;46:834–841. doi: 10.1002/1531-8249(199912)46:6<834::aid-ana5>3.0.co;2-m. doi:10.1002/1531-8249(199912)46:6<834::AID-ANA5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Reddick WE, Russell JM, Glass JO, et al. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn Reson Imaging. 2000;18:787–793. doi: 10.1016/s0730-725x(00)00182-x. doi:10.1016/S0730-725X(00)00182-X. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SL, Reddick WE, Glass JO, Gajjar A, Goloubeva O, Mulhern RK. Decline in corpus callosum volume among pediatric patients with medulloblastoma: longitudinal MR imaging study. Am J Neuroradiol. 2002;23:1088–1094. [PMC free article] [PubMed] [Google Scholar]
- 18.Reddick WE, Glass JO, Palmer SL, et al. Atypical white matter volume development in children following craniospinal irradiation. Neuro Oncol. 2005;7:12–19. doi: 10.1215/S1152851704000079. doi:10.1215/S1152851704000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19:472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- 20.Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–2519. doi: 10.1002/cncr.11355. doi:10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 21.Khong PL, Kwong DL, Chan GC, Sham JS, Chan FL, Ooi GC. Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: a pilot study. Am J Neuroradiol. 2003;24:734–740. [PMC free article] [PubMed] [Google Scholar]
- 22.Khong PL, Leung LH, Chan GC, et al. White matter anisotropy in childhood medulloblastoma survivors: association with neurotoxicity risk factors. Radiology. 2005;236:647–652. doi: 10.1148/radiol.2362041066. doi:10.1148/radiol.2362041066. [DOI] [PubMed] [Google Scholar]
- 23.Khong PL, Leung LH, Fung AS, et al. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. doi:10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 24.Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro Oncol. 2006;8:244–252. doi: 10.1215/15228517-2006-002. doi:10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer SL, Reddick WE, Glass JO, et al. Regional white matter anisotropy and reading ability in patients treated for pediatric embryonal tumors. Brain Imaging Behav. 2010;4:132–140. doi: 10.1007/s11682-010-9092-1. doi:10.1007/s11682-010-9092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aukema EJ, Caan MW, Oudhuis N, et al. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiat Oncol Biol Phys. 2009;74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. doi:10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- 27.Qiu D, Kwong DL, Chan GC, Leung LH, Khong PL. Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: reflection of regional white matter radiosensitivity? Int J Radiat Oncol Biol Phys. 2007;69:846–851. doi: 10.1016/j.ijrobp.2007.04.041. doi:10.1016/j.ijrobp.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 28.Rueckriegel SM, Driever PH, Blankenburg F, Ludemann L, Henze G, Bruhn H. Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int J Radiat Oncol Biol Phys. 2010;76:859–866. doi: 10.1016/j.ijrobp.2009.02.054. doi:10.1016/j.ijrobp.2009.02.054. [DOI] [PubMed] [Google Scholar]
- 29.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. doi:10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 31.Woodcock RW, McGrew KS, Mather N. Woodcock–Johnson III: Tests of Achievement. Itasca, IL: Riverside; 2001. [Google Scholar]
- 32.Strauss E, Sherman EM, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 33.Conners CK. Conners’ Continuous Performance Test II. Noth Tonawanda, NY: Multi-Health Systems, Inc.; 2001. [Google Scholar]
- 34.Wechsler D. Wechsler Adult Intelligence Scale–Third Edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 35.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test–Second Edition. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- 36.Warrington EK. Recognition Memory Test Manual. Windsor, UK: NFER-Nelson; 1984. [Google Scholar]
- 37.Reitan R. The Halstead–Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2nd ed. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 38.Meyers J, Meyers K. The Meyers Scoring System of the Rey Complex Figure and the Recognition Trial: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 39.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test–64 Card Version. Lutz, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 40.Benton AL, Hamsher KD, Sivan AB. Multilingual Aphasia Examination. 3rd ed. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- 41.Golden CJ, Freshwater SM. Stroop Color and Word Test: Revised Examiner's Manual. Wood Dale, IL: Stoelting Co.; 2002. [Google Scholar]
- 42.Roth RM, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function–Adult Version. Lutz, FL: Psychological Assessment Resources, Inc.; 2005. [Google Scholar]
- 43.Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain. 2012;135:1154–1164. doi: 10.1093/brain/aws021. doi:10.1093/brain/aws021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barahmani N, Carpentieri S, Li XN, et al. Glutathione S-transferase M1 and T1 polymorphisms may predict adverse effects after therapy in children with medulloblastoma. Neuro Oncol. 2009;11:292–300. doi: 10.1215/15228517-2008-089. doi:10.1215/15228517-2008-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brackett J, Krull KR, Scheurer ME, et al. Antioxidant enzyme polymorphisms and neuropsychological outcomes in medulloblastoma survivors: a report from the Childhood Cancer Survivor Study. Neuro Oncol. 2012;14:1018–1025. doi: 10.1093/neuonc/nos123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butler RW, Copeland DR, Fairclough DL, et al. A multicenter, randomized clinical trial of a cognitive remediation program for childhood survivors of a pediatric malignancy. J Consult Clin Psychol. 2008;76:367–378. doi: 10.1037/0022-006X.76.3.367. doi:10.1037/0022-006X.76.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardy KK, Willard VW, Bonner MJ. Computerized cognitive training in survivors of childhood cancer: a pilot study. J Pediatr Oncol Nurs. 2011;28:27–33. doi: 10.1177/1043454210377178. doi:10.1177/1043454210377178. [DOI] [PubMed] [Google Scholar]
- 48.Patel SK, Katz ER, Richardson R, Rimmer M, Kilian S. Cognitive and problem solving training in children with cancer: a pilot project. J Pediatr Hematol Oncol. 2009;31:670–677. doi: 10.1097/MPH.0b013e3181b25a1d. doi:10.1097/MPH.0b013e3181b25a1d. [DOI] [PubMed] [Google Scholar]
- 49.Kesler SR, Lacayo NJ, Jo B. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Inj. 2011;25:101–112. doi: 10.3109/02699052.2010.536194. doi:10.3109/02699052.2010.536194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. doi:10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engvig A, Fjell AM, Westlye LT, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. [published online ahead of print August 5, 2011]. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21370. doi:10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]