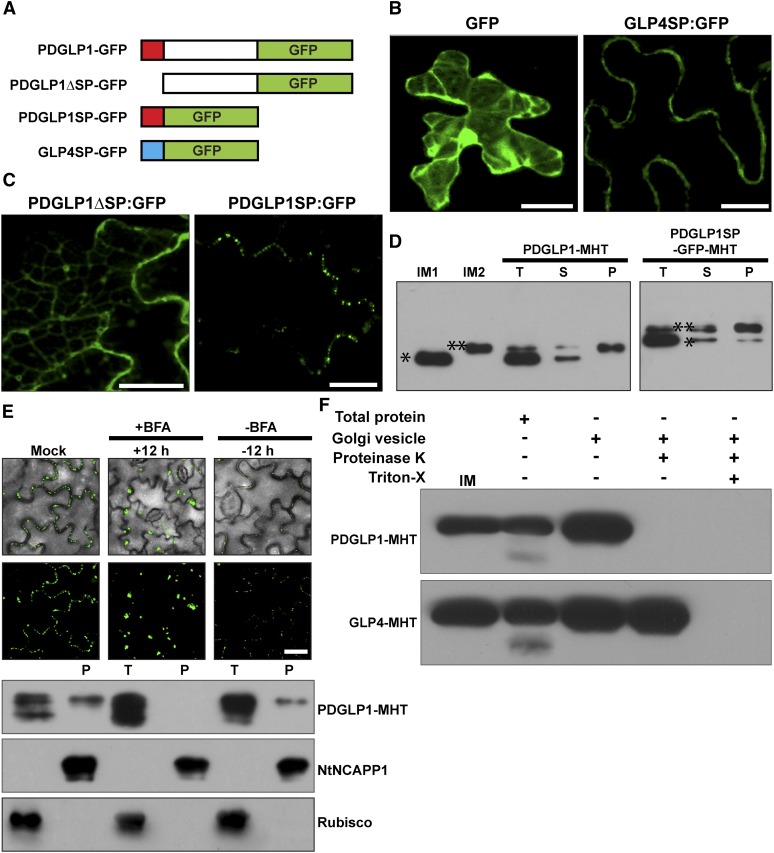
Figure 4.
The PDGLP1 SP Is Necessary and Sufficient for Protein Delivery to PD via the ER-Golgi Secretory Pathway.
(A) Arabidopsis PDGLP1 and GLP4 constructs used to test the role of the SP in protein targeting to PD. Putative N-terminal SPs shown as a red (PDGLP1) and blue (GLP4) box. GFP was fused to the C terminus of PDGLP1, a SP-deleted PDGLP1 mutant (PDGLP1ΔSP-GFP), and alone with the PDGLP1 SP (PDGLP1SP-GFP) or the GLP4 SP (GLP4SP-GFP).
(B) The control constructs tested remained either in the cytoplasm and nucleus (GFP alone) or in the cell wall (GLP4SP-GFP).
(C) The PDGLP1ΔSP-GFP mutant is no longer targeted to PD, whereas the PDGLP1SP-GFP construct accumulated in punctate foci along the cell wall, likely representing location to PD. In (B) and (C), the tested constructs were introduced into N. benthamiana leaves by particle bombardment and observed using confocal microscopy after a 24-h incubation period. Bars = 10 µm.
(D) PDGLP1 SP is not cleaved during protein targeting to PD. PDGLP1-MHT and PDGLP1SP-GFP-MHT were first agroinfiltrated in N. benthamiana leaves and then 4 d later leaf tissues were extracted for biochemical experiments. IM1 and IM2, internal markers for PDGLP1 lacking (single asterisk) or containing (double asterisk) the SP; P, PECP fraction; S, soluble protein fraction; T, total protein fraction.
(E) PDGLP1 uses the secretory pathway for its targeting to PD. A PDGLP1-GFP construct was agroinfiltrated into N. benthamiana leaves and, 24 h later, they were imaged by confocal microscopy prior to infiltration with BFA. Following a 12-h BFA treatment, recovery experiments were performed by infiltration of a 0.5% (v/v) DMSO solution. A parallel set of experiments was performed with a PDGLP1-MHT construct, and these leaves were employed to test for the effect of BFA treatment on PDGLP1-GFP subcellular localization. Here, Nt-NCAPP1 and Rubisco antibodies were used to confirm the purity of the PECP fraction. P, PECP fraction; T, total protein fraction. Bar = 10 µm.
(F) PDGLP1 is located on the outside of the Golgi-derived vesicles. PDGLP1-MHT and GLP4-MHT constructs were agroinfiltrated into N. benthamiana leaves and Golgi-derived vesicles were fractionated by velocity sedimentation. Vesicles were either treated with Proteinase K (20 µg/mL) or first pretreated with 0.1% Triton X-100 followed by Proteinase K. Note: PDGLP1-MHT was not detected in Proteinase K–treated vesicles, whereas a combination of Triton X-100 and Proteinase K was necessary to eliminate the GLP4-MHT signal.
[See online article for color version of this figure.]