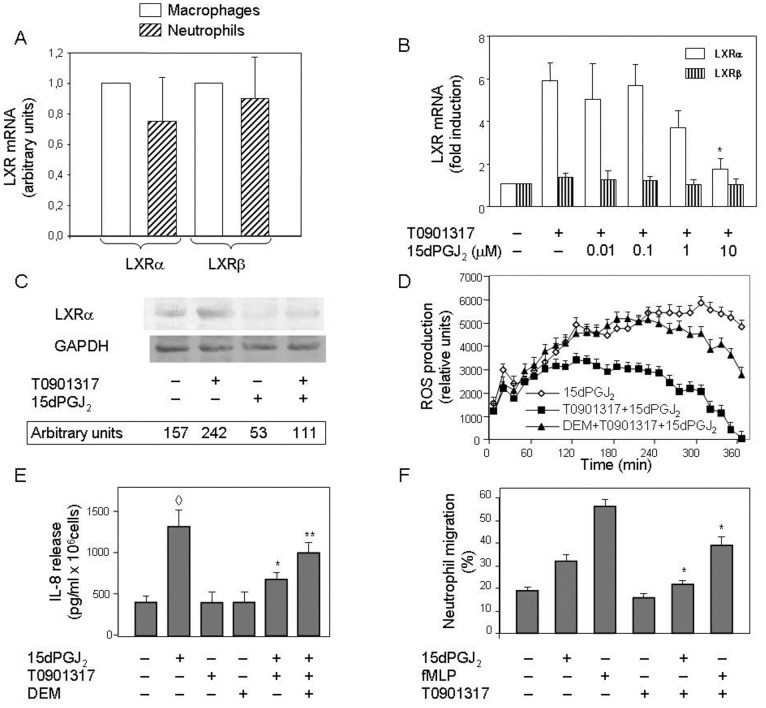
Figure 1. 15dPGJ2 prevents ligand-induced LXRα mRNA expression in a dose-dependent manner.
Neutrophils or macrophages were left untreated (A), or else neutrophils were preincubated at 37°C for 1 h with or without 15dPGJ2 at the indicated doses (B) or at a 10 µM concentration (C), and then they were treated or not with T0901317 for 18 h (B and C). Then LXRα and LXRβ mRNA levels were measured by real time RT-PCR (A and B), or LXRα protein levels were analyzed by Western blotting (C). Statistical data from real time RT-PCR experiments were normalized to LXRα or LXRβ levels found in human macrophages (A) and corrected for differences in β-actin mRNA levels (as endogenous gene) and expressed as fold induction (A and B). GAPDH bands in Western blots are shown for the sake of protein loading controls (C). In a different set of experiments, neutrophils were pretreated or not with 10 µM DEM for 30 min (D and E) and, after preincubation in the absence or presence of 1 µM T0901317 for 4 h (D and E), 10 µM 15dPGJ2 was added and ROS levels were monitored by luminescence measurement for the next 6 h (D), or released IL-8 levels were quantitated by ELISA after an incubation period of 4 h (E). Neutrophil migration was quantitated in cells preincubated with or without 1 µM T0901317 for 4 h and then transferred to Transwell chambers and treated with 10 µM 15dPGJ2 or 0.1 µM fMLP for 1 h. Results are expressed as the percentage of cells that migrated from the upper to the lower compartment (F). Each panel is representative of a set of three experiments yielding similar results. Values are plotted as the mean ± SEM (n = 3) (A, B, D, E and F). * P<0.01 for T0901317-stimulated, 15dPGJ2 (B, F) or fMLP (F)-treated versus -untreated. ◊ P<0.01 for 15dPGJ2-treated versus untreated (E). ** P<0.01 for 15dPGJ2 and T0901317-stimulated, DEM-treated versus -untreated (E).