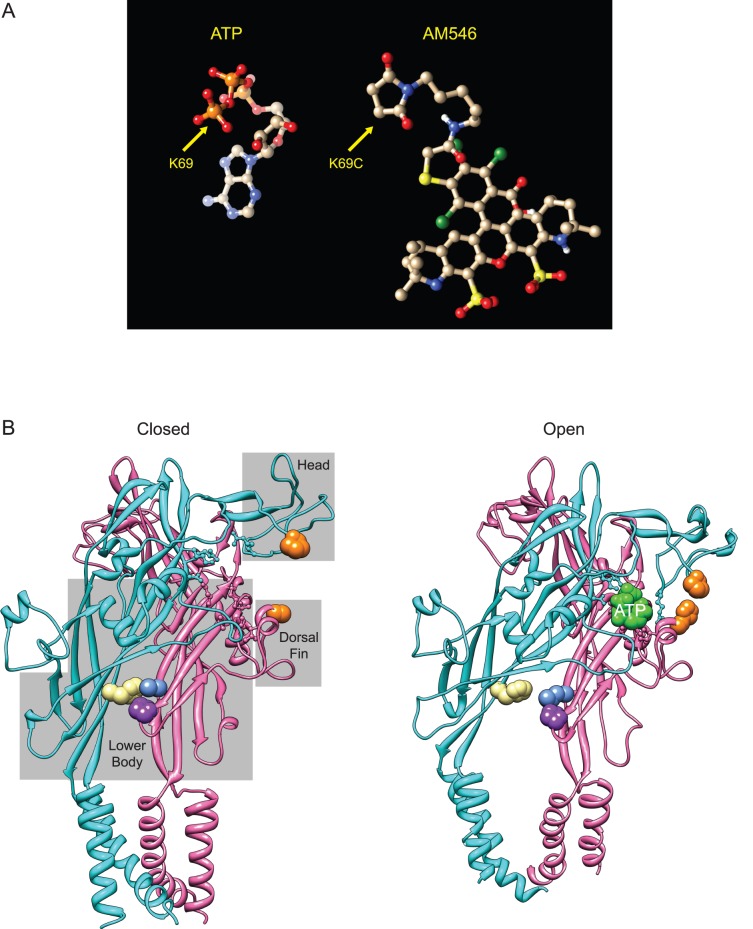
Figure 10. Structural features likely contributing to activation of AM546 modified K69C channels by zinc or pH.
A: Structure of ATP folded as it sits in the binding site of zebrafish P2X4.1, and structure of AM546 with the linker folded back upon itself. B: Structure of P2X4.1 in the closed and open (ATP-bound) states. Only two of the three subunits are illustrated. Residues that bind ATP are shown in ball and stick format. The gray boxes superimposed on the closed state indicate domains of the receptor named according to the dolphin model of Kawate and Gouaux [32]. Left: The closed state structure of zP2X4.1 (PDB 4DW0) with positions homologous to key residues of rP2X2 colored. Orange indicates the location of the histidines of the potentiating zinc binding site of rP2X2, which are P125 (cyan subunit) and H219 (pink subunit) in zP2X4.1. Beige is the location of F327 (of the cyan subunit), which is homologous to rP2X2 H319. Blue (L64) and purple (P199) indicate the residues (of the pink subunit) that bind to F327 in the closed state. Right: The ATP bound open state structure of zP2X4.1 (PDB 4DW1). Residues are colored as in A. The ATP at the interface between the two illustrated subunits is also shown (green). Note that the side group of H219 has been rotated from its position in 4DW1 to emphasize the close apposition of the orange histidines that is possible in rP2X2 when zinc is present.