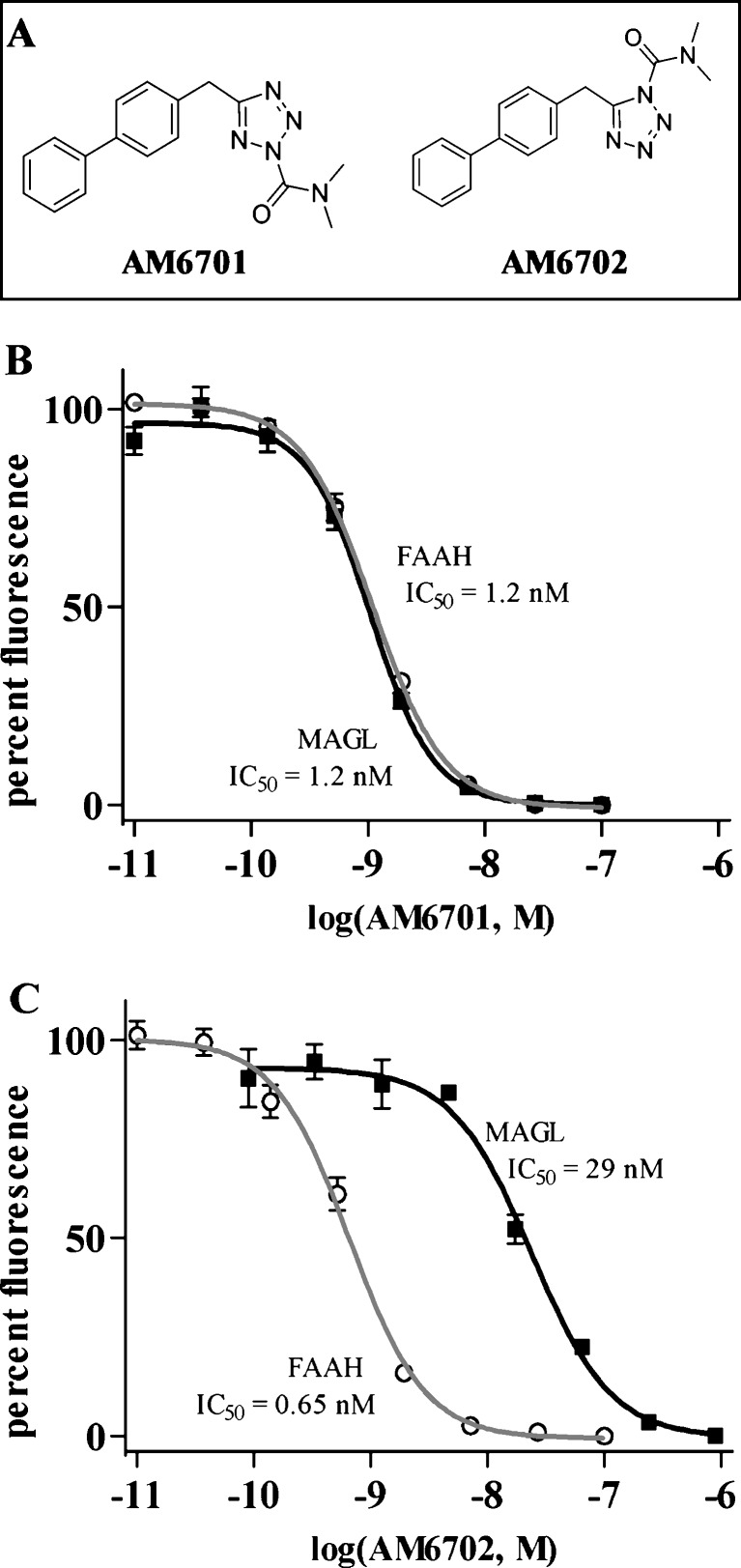
Fig. 1.
Assessment of fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) inhibition. The structures of the carbamoyl tetrazole compounds used in this study are shown (a): 5-([1,1'-biphenyl]-4-ylmethyl)-N,N-dimethyl-2H-tetrazole-2-carboxamide (AM6701) and 5-([1,1'-biphenyl]-4-ylmethyl)-N,N-dimethyl-1H-tetrazole-1-carboxamide (AM6702). Recombinant FAAH and MAGL were treated with increasing concentrations of AM6701 (b) and AM6702 (c) in triplicate in independent experiments, and the inhibitory activities of the compounds were determined. Enzyme activity data were normalized to 100% fluorescence generation in the absence of drugs and shown as means ± standard deviation. In the FAAH and MAGL assays, AM6701 caused a concentration-dependent inhibition of both enzymes and demonstrated equal potency for FAAH (IC50 = 1.2 ± 0.13 nM) and MAGL (IC50 = 1.2 ± 0.35 nM). AM6702 caused a concentration-dependent inhibition of both activities, but more potently inhibited FAAH in comparison to MAGL. AM6702 demonstrated a 44-fold selectivity for FAAH (IC50 = 0.65 ± 0.007 nM) compared to MAGL (IC50 = 29 ± 5.9 nM)