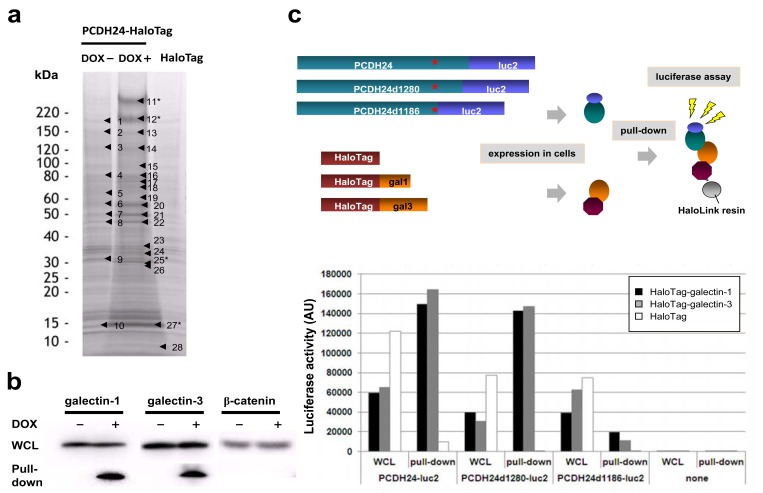
Fig. (2). Pull-down analysis for PCDH24-interacting proteins.
(a) SDS-PAGE of pull-down samples by HaloLink™ Resin from HCT116-PCDH24-HaloTag cells cultured with or without DOX and HCT116 cells ectopically expressing HaloTag. The samples were concentrated, then resolved by 5–20% SDS-PAGE and subjected to imida-zole-zinc reverse staining. The excised bands subjected to LC-MS/MS analysis are highlighted by black arrowheads. The numerals of the proteins analyzed in this study are indicated with asterisks (i.e., #11 and #12: PCDH24; #25: galectin-3; #27: galectin-1). The proteins identi-fied by the pull-down assay and MS analysis are shown in Supplemental Table 1. (b) Western blot analysis of the pull-down samples. West-ern blot experiments were performed for whole cell lysate (WCL) and pull-down assay samples using anti-galectin-1, anti-galectin-3 and anti-β-catenin antibodies. (c) Delineation of the galectin-interaction domain in the intracellular region of PCDH24. Schematic representa-tion of the luciferase-based pull-down assay is shown. Expression clones for luciferase-fused full-length PCDH24 (PCDH24-luc2, amino acids 1–1310 residues) and the C-terminal deletion mutants (PCDH24d1280-luc2, amino acids 1–1280; PCDH24d1186-luc2, amino acids 1–1186) were co-transfected with HaloTag, HaloTag-fused galectin-1 or HaloTag-fused galectine-3 expression clones into HCT116 cells. The amounts of luciferase-fusion proteins in whole cell lysates (WCL) and the pull-down samples were measured as the luciferase activity with the Dual-Glo™ substrate using a GloMAX™ luminometer (upper panel). Results of the experiments are indicated as an arbitrary unit of the luciferase activities. Transfection of the HaloTag-containing clone without the PCDH24 clones (none) was performed as a negative control (lower panel).