Table 8.
Scope of the Cross-Coupling of Enantioenriched 22 with (Hetero)Aryl Chlorides
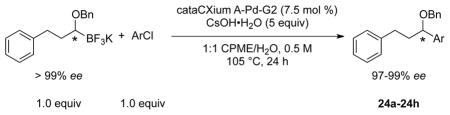
| |||||
|---|---|---|---|---|---|
| Entry | (x)-22 | ArCl | % ee (x) | Isolated Yield (%) | |
| 1 | (S) |
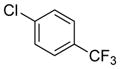
|
24a | 99 (R) | 81 |
| 2 | (S) |
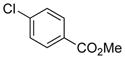
|
24b | > 99 (R) | 62a |
| 3 | (S) |

|
24e | > 99 (R) | 70 |
| 4 | (S) |
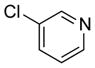
|
24h | > 99 (R) | 86 |
| 5 | (S) |

|
24c | 99 (R) | 75 |
| 6 | (R) |
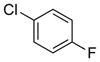
|
24d | 97 (S) | 77 |
| 7 | (R) |
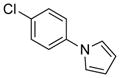
|
24f | 97 (S) | 78 |
| 8 | (R) |
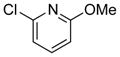
|
24g | 97 (S) | 65 |
Reaction conditions: 1.0 equiv of trifluoroborate, 1.0 equiv of electrophile, 7.5 mol % Pd, 5.0 equiv of base, 1:1 CPME/H2O, 105 °C, 24 h.
Reaction with 5 equiv of Cs2CO3.
