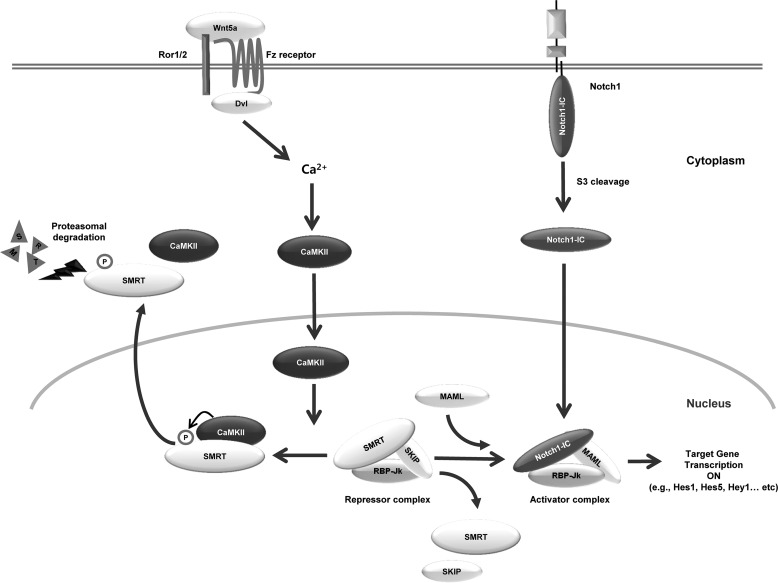
FIGURE 8.
Schematic diagram of CaMKII regulates Notch1 signaling through proteasomal degradation of SMRT. Frizzled (Fz), and Ror1/2 that mediates Wnt5a/Ca2+ signaling has not yet been elucidated, however, the formation of a trimeric complex with Fz and Ror1/2 and the PDZ domain-mediated binding with dishevelled (Dvl) could be important. In this study, we evaluated that the mechanism by which Wnt up-regulates Notch1 target genes. We demonstrated that Wnt5a induced CaMKII enhances Notch transcriptional activity. CaMKII physically interacts with SMRT and disrupts the SMRT-RBP-Jk transcription complex through degradation of SMRT. Dissociation of SMRT from RBP-Jk induced by CaMKII activates Hes1 and Hes5 transcriptional activity. Furthermore, CaMKII can phosphorylate SMRT and activate the Notch1 signaling pathway. The kinase activity of CaMKII is necessary for up-regulation of the transcription activity of Notch1 target genes. The results of this study suggest that CaMKII is a crucial regulator for signal cross-talk between Wnt5a and Notch signaling via down-regulation of the SMRT protein level.