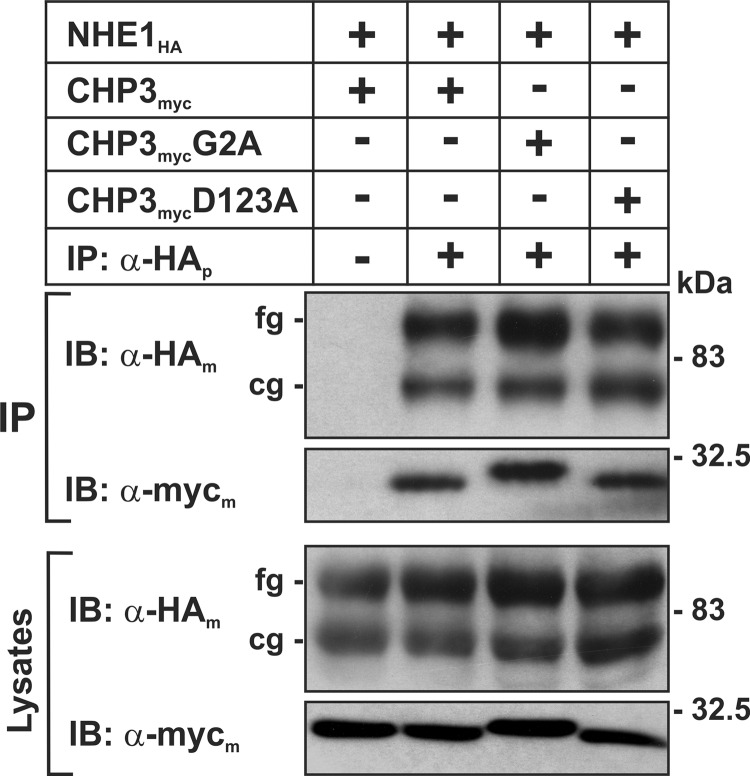
FIGURE 1.
N-Myristoylation and Ca2+ binding-defective mutants of CHP3 form a complex with NHE1 in transfected cells. Chinese hamster ovary AP-1 cells stably expressing NHE1HA were transiently transfected with either wild-type or mutant constructs of CHP3myc that are defective in either N-myristoylation (G2A) or calcium-binding (D123A). Twenty four hours post-transfection, cell lysates were prepared, and NHE1HA-containing protein complexes were immunoprecipitated with a rabbit polyclonal antibody specific to the HA epitope (α-HAp). The cell lysates and immunoprecipitates (IP) were fractionated by SDS-PAGE and analyzed by immunoblotting (IB) using mouse monoclonal antibodies specific to either the HA or Myc epitopes (α-HAm or α-Mycm, respectively). The two immunoreactive bands visualized in the NHE1HA blots represent the immature core-glycosylated (cg) and mature fully glycosylated (fg) forms of the exchanger. Data shown are representative of three separate experiments.