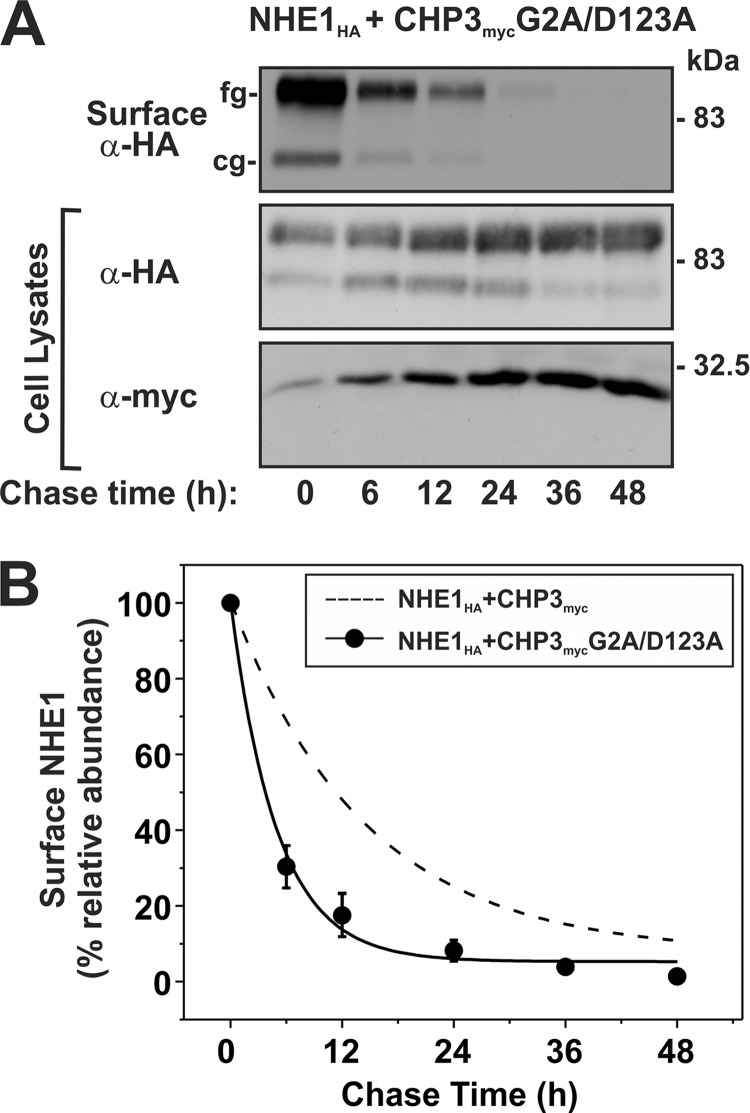
FIGURE 10.
Loss of both N-myristoylation and Ca2+ binding of CHP3 decreases cell surface stability of NHE1. AP-1 cells stably expressing NHE1HA and the combined N-myristoylation- and Ca2+ binding-defective CHP3 mutant (G2A/D123A) were seeded equally in a 6-well plate and cultured to subconfluence. A, surface proteins were biotinylated for 30 min on ice, and after removal of excess biotin, cells were returned to regular growth media supplemented with 10% FBS and antibiotics and incubated at 37 °C, 5% CO2 in humidified atmosphere. At the indicated time points, cell lysates were obtained and, after removal of a small fraction for Western blotting, were subject to incubation overnight at 4 °C with NeutrAvidinTM-agarose beads to extract surface-labeled NHE1HA. Proteins were separated by SDS-PAGE and immunoblotted to visualize the remaining surface NHE1HA as a function of time as described in the legend to Fig. 8. B, intensities of the immunoreactive signals for surface NHE1HA were measured by densitometry using ImageJTM software, normalized to maximum expression at time 0 h, and plotted as a function of time. For comparative purposes, the plot was overlaid with the data describing the effect of wild-type CHP3 on NHE1 cell surface stability shown in Fig. 8.