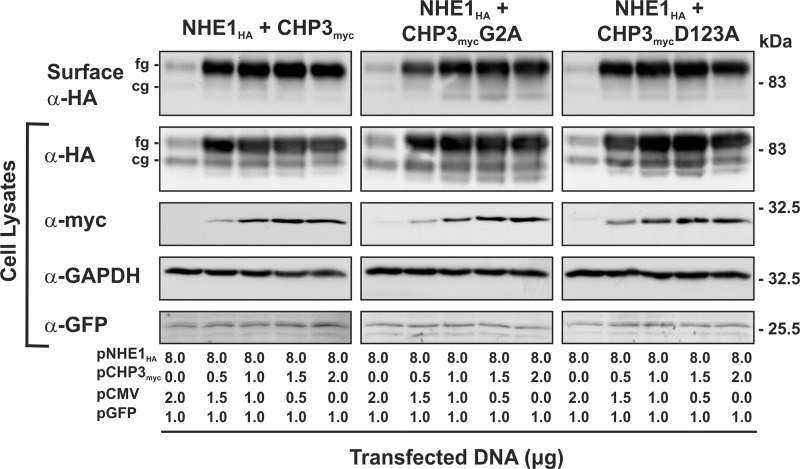
FIGURE 6.
CHP3-mediated trafficking of NHE1 to the cell surface is not dependent on N-myristoylation or Ca2+ binding of CHP3. AP-1 cells were grown to subconfluence on 10-cm dishes and then transiently transfected with a fixed amount of expression plasmid DNA containing NHE1HA (8 μg) and an increasing ratio of CHP3myc (WT, G2A, or D123A) to empty expression vector (pCMV) (0–2 μg per dish). Cells were also simultaneously cotransfected with a plasmid that expresses green fluorescent protein (pGFP) as a control for transfection efficiency. At 48 h post-transfection, cells were subjected to surface biotinylation as described under “Experimental Procedures,” and whole cell lysates were prepared. A major fraction of the lysates was subsequently incubated with NeutrAvidin-Sepharose beads to isolate the biotinylated cell surface proteins. Aliquots of the whole cell lysates and biotinylated cell surface proteins were subjected to SDS-PAGE and immunoblotting. Expression of fully glycosylated (fg) and core-glycosylated (cg) forms of NHE1HA and CHP3myc were detected as described in Fig. 4. GFP was detected using a primary rabbit polyclonal anti-GFP antibody (α-GFP) and a secondary goat anti-rabbit antibody conjugated to horseradish peroxidase. Immunoblots of the lysates were stripped and reprobed for endogenous GAPDH as a control for protein loading. Data shown are representative of three independent experiments.