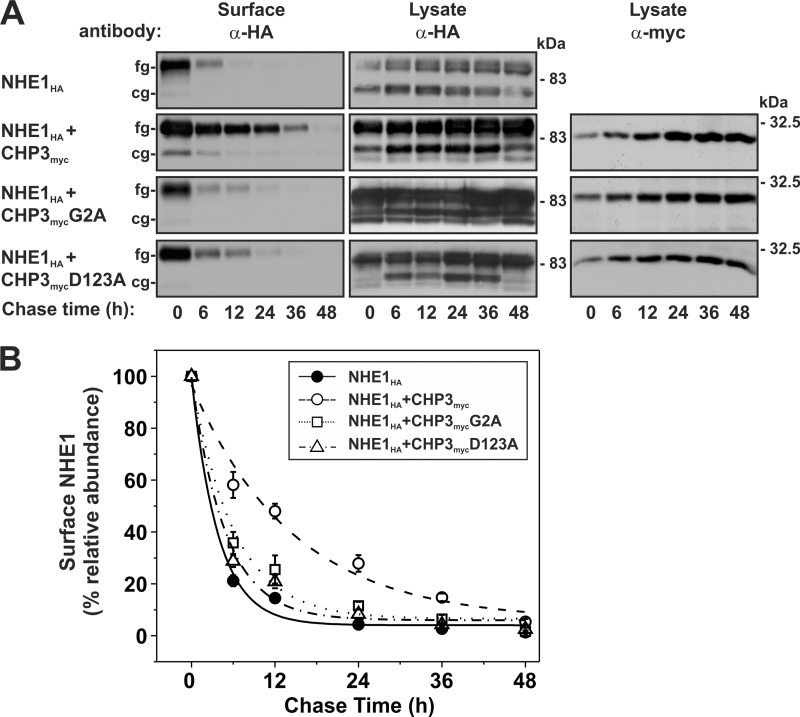
FIGURE 8.
N-Myristoylation and Ca2+ binding of CHP3 enhance cell surface stability of NHE1. A, AP-1 cells stably expressing NHE1HA or stably coexpressing NHE1HA and individual variants of CHP3myc (WT, G2A, and D123A) were subject to cell surface biotinylation, as described under “Experimental Procedures.” The cells were returned to growth media at 37 °C, and then cell lysates were prepared at varying times over a 48-h period. At each time point, a small fraction of the cell lysates was removed for immunoblotting, and the remainder was incubated with NeutrAvidin-Sepharose beads to extract the biotinylated proteins. Total cellular levels of core-glycosylated (cg) and fully (fg) glycosylated NHE1HA and CHP3myc as well as levels of surface-biotinylated, fully glycosylated NHE1HA were monitored as a function of time by SDS-PAGE and immunoblotting, as described in the legend to Fig. 4. It was noted that occasionally a small amount of the core-glycosylated NHE1HA was detected in the cell surface biotinylated fraction, possibly indicating contamination from intracellular compartments. However, when the blots were stripped and reprobed for intracellular GAPDH, no signal was detected (data not shown). This suggests that a minor fraction of the core-glycosylated NHE1 can traffic to the plasma membrane, perhaps as a consequence of overexpression. B, data represent densitometric analysis of the cell surface fully glycosylated NHE1HA presented in A, normalized as a percentage of its maximal abundance at time 0 h and plotted as a function of time. Values represent the mean ± S.E. of three experiments. Error bars smaller than the symbol are absent.