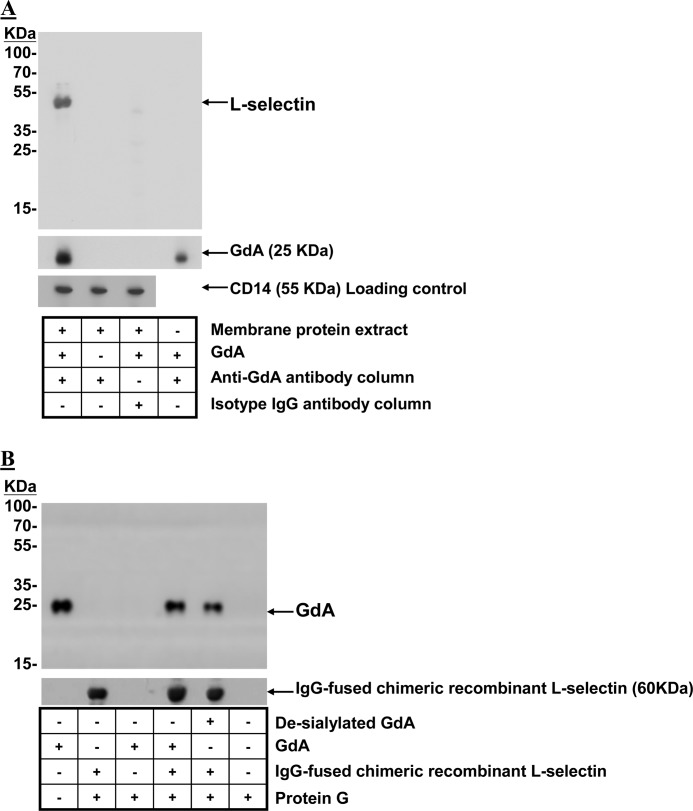
FIGURE 6.
Interaction between GdA and L-selectin. A, GdA-treated membrane protein extracts from monocytes were co-immunoprecipitated with anti-glycodelin antibody-conjugated Sepharose beads. Western blotting was performed on the eluted fractions with anti-L-selectin and anti-glycodelin antibodies. Membrane protein extracts without GdA treatment or co-immunoprecipitated with isotope antibody-conjugated Sepharose beads after GdA treatment served as controls. Equal loading of membrane protein extracts onto the Sepharose beads was confirmed by Western blotting of the flow-through fractions using anti-CD14 antibodies. Representative Western blots from four individual experiments are shown. B, IgG-fused recombinant human L-selectin chimeric proteins were incubated with native or desialylated GdA in PBS at 4 °C. Protein G-Sepharose beads were used to precipitate the GdA-recombinant L-selectin complex. The captured complex was resolved by SDS-PAGE and analyzed by Western blotting using anti-L-selectin or anti-glycodelin antibody. Representative Western blots from four individual experiments are shown.