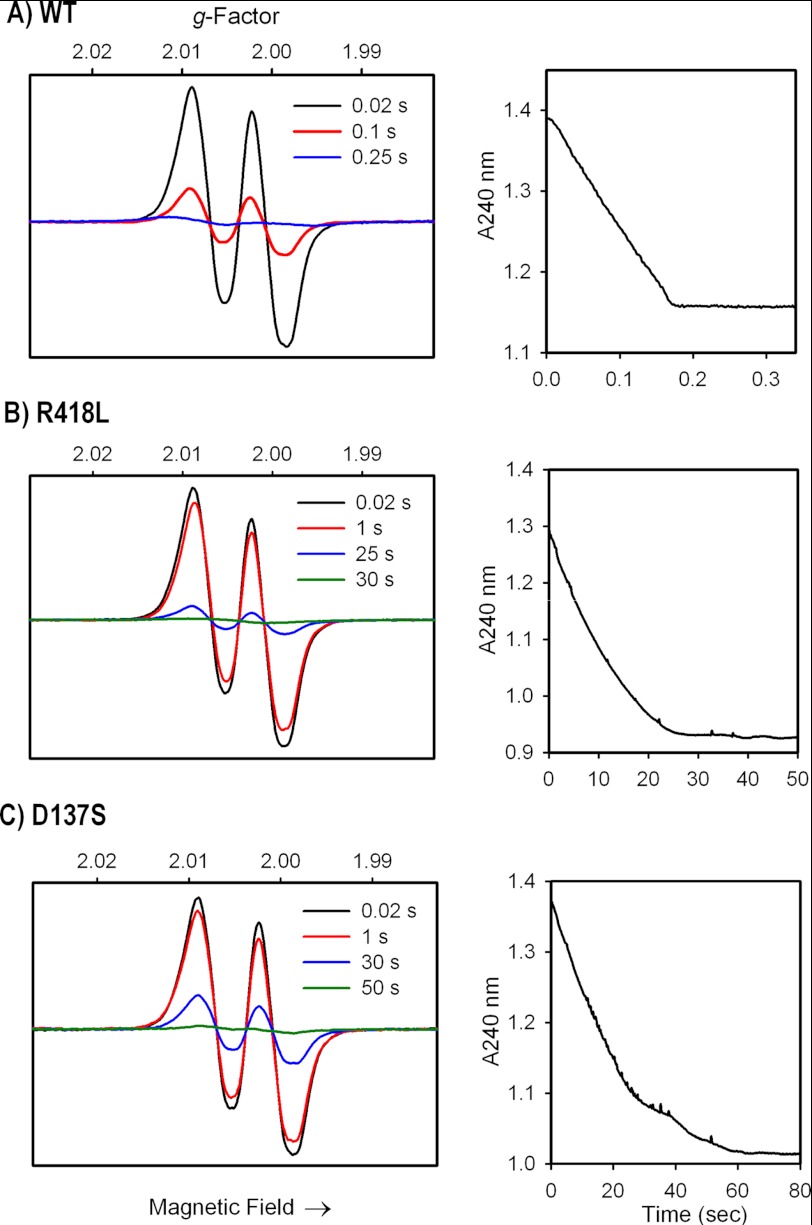
FIGURE 2.
MYW-radical formation and H2O2 consumption in A) WT KatG; B) KatG[R418L]; C) KatG[D137S] mutants. Left panels: rapid freeze quench X-band EPR spectra (77 K) of enzymes (50 μm) reacted with 1000-fold molar excess of H2O2 at pH 7.2. Samples were frozen at the indicated times after mixing at room temp. Spectra are plotted on the same signal intensity scales. The narrow doublet signal contains one strong and one weak hyperfine coupling interaction (aH1, H2 = 11 and ∼2.5 Gauss) arising from the β-methylene hydrogens of residue Tyr-229 (13). Right panels: stopped-flow spectrophotometric time courses showing H2O2 consumption monitored at 240 nm under the same conditions used for the EPR experiments.