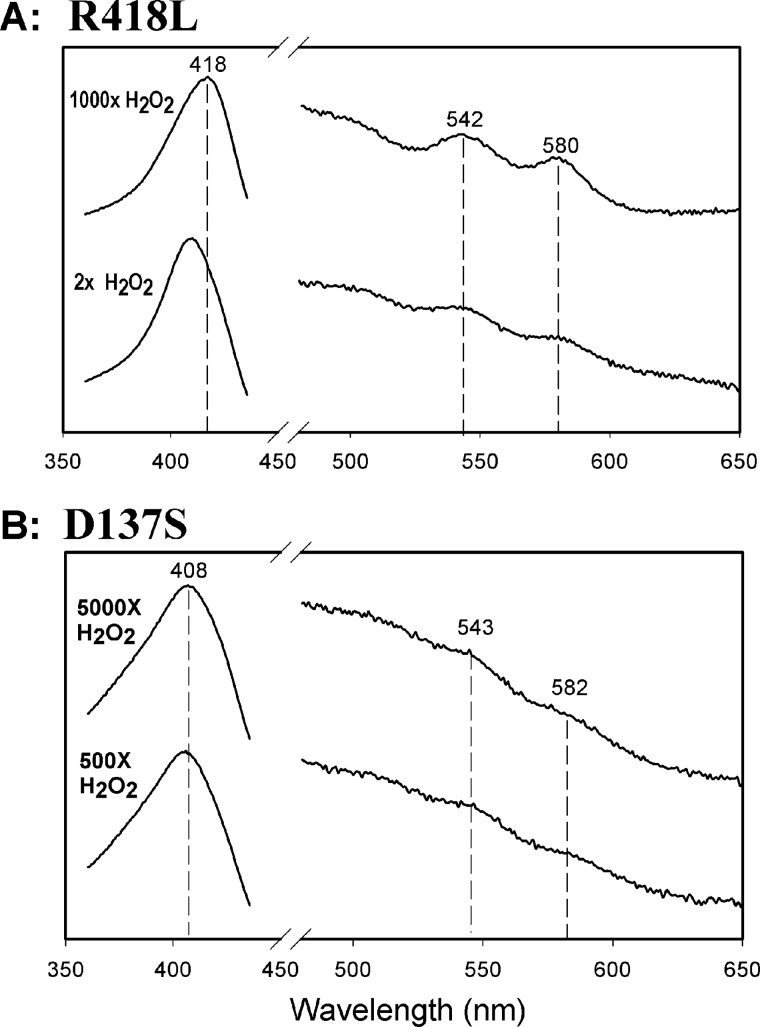
FIGURE 3.
Dioxyheme formation in KatG[R418L] and KatG[D137S]. Spectra were recorded 4 ms after mixing H2O2 with KatG[R418L] (A) or KatG[D137S] (B). Enzyme concentrations were 10 μm (final) and the molar equivalents of H2O2 were as indicated. All reactions were performed in 20 mm potassium phosphate buffer, pH 7.2 at room temperature using a stopped-flow spectrophotometer. The peaks in the visible wavelength region through which lines are drawn closely match those assigned to typical peroxidase Compound III and indicate the dioxyheme component in both reactions.