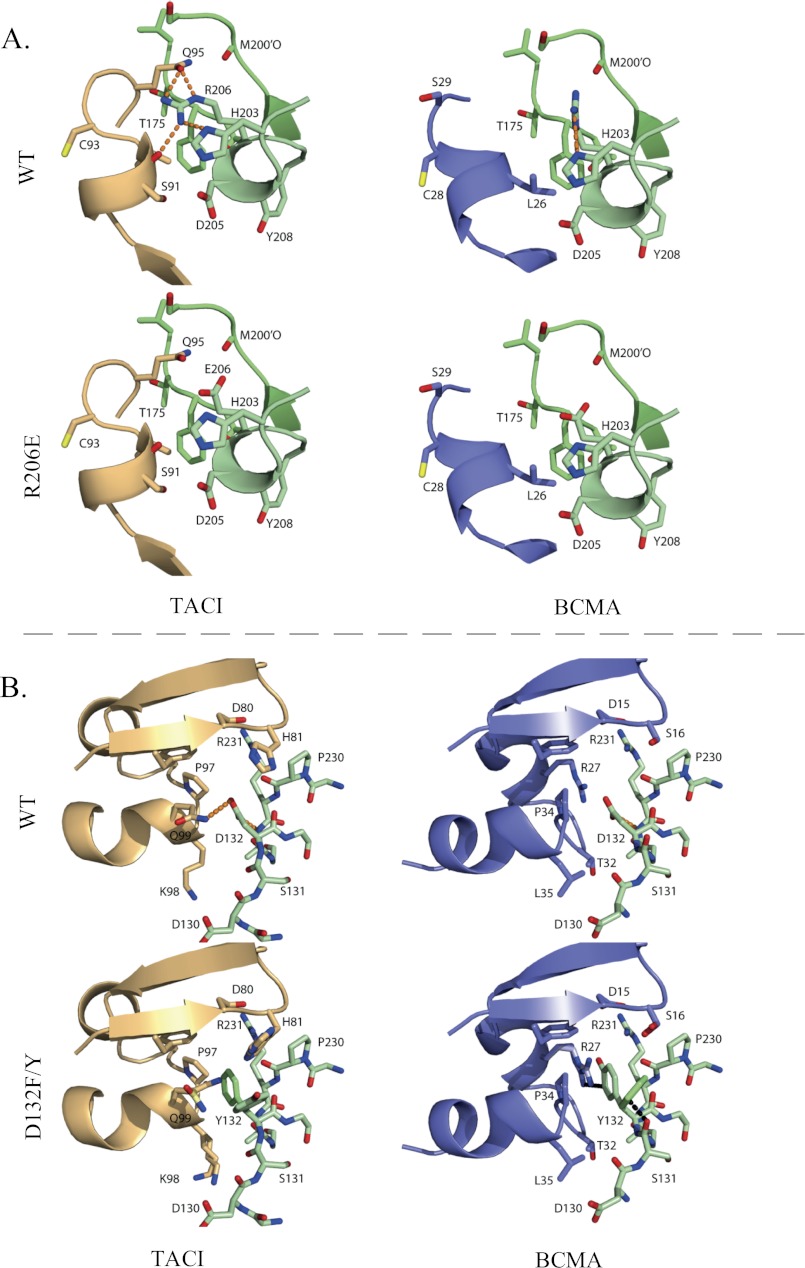
FIGURE 6.
A, structural consequences of the R206E substitution. In the WT APRIL-TACI crystal structure, Arg-206 makes hydrogen bonds with TACI, whereas in the WT APRIL-BCMA crystal structure, Arg-206 is not involved in the interaction with BCMA. The Glu-206 substitution in TACI and BCMA is not involved in hydrogen bond interactions. B, structural consequences of the D132F and D132Y substitution. In the WT APRIL-BCMA structure, Asp-132 (D132) is involved in a favorable electrostatic interaction with Arg-27, whereas in the WT APRIL-TACI complex, Asp-132 accepts a (weak) hydrogen bond from Gln-99 (Q99) of TACI. The loss of this hydrogen bond due to the Phe-132 and Tyr-132 substitution is compensated in TACI by favorable Van der Waals interactions, whereas in BCMA, the Phe or Tyr either clashes with Arg-27 (R27) or with the main chain oxygen of Ser-131 (S131) of APRIL. TACI is depicted in orange, BCMA in blue, and APRIL in green. The D132F and D132Y structures are superimposed; residues that differ are indicated in lighter shades of blue, green, and orange. Hydrogen bonds are shown as an orange dotted line, and Van der Waals clashes are shown as a black dotted line.