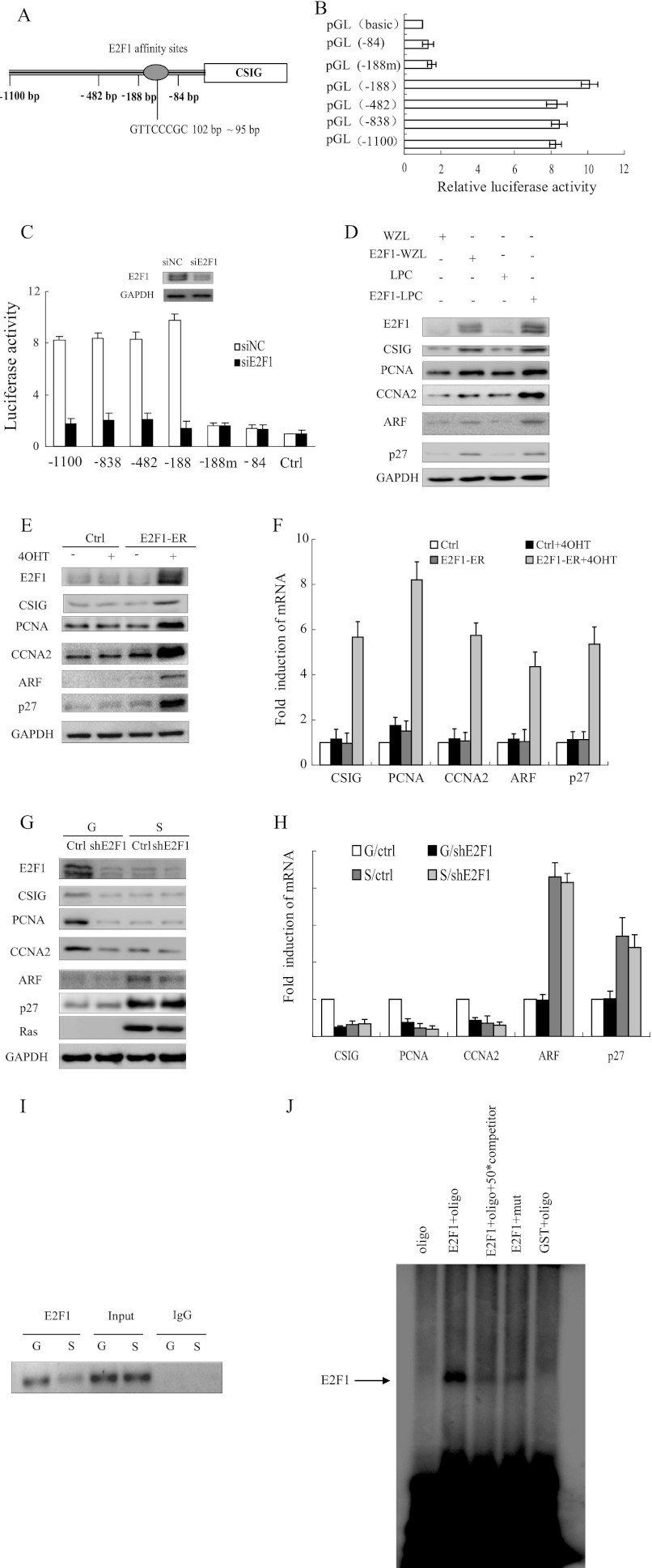
FIGURE 1.
CSIG is one of the target genes of E2F1. A, schematic representation of E2F1 affinity sites. B, different constructs were analyzed for luciferase expression in HeLa cells. The values represent the means and standard errors of three independent experiments. C, deletion mutants of human CSIG promoter constructs were cotransfected with siE2F1 oligonucleotides and small interference nonsense control (siNC) in HeLa cells and were analyzed for luciferase expression. The values represent the means and standard errors from three independent experiments. D, 2BS cells stably expressing retroviral E2F1 expression plasmids were analyzed for the indicated proteins 7 days after retroviral transduction. E and F, the 2BS cells stably expressing the hormone-regulated E2F1-ER fusion construct were treated with 100 nm 4-hydroxytamoxifen (4OHT) for 18 h, and the protein and mRNA levels of the indicated genes were determined by Western blot and quantitative RT-PCR. The values represent the means and standard errors from three independent experiments. G and H, the 2BS cells expressing vector alone (G) or a combination of pWZL-Ras-Hygro (S), and vector (Ctrl) or shRNA against E2F1 (shE2F1) were harvested to determine the protein and mRNA levels of the indicated genes by Western blot and quantitative RT-PCR. The values represent the means and standard errors from three independent experiments. I, growing (G) and Ras-induced senescent (S) 2BS cells were processed for ChIP using an anti-E2F1 antibody (C-20x; Santa Cruz Biotechnology). The primer set used includes the region from −255 to −35 bp of the CSIG promoter. J, EMSA was performed using E2F1 protein expressed in vitro through the GST expression system. oligo, end-labeled oligonucleotide probes containing the E2F1 binding site on the CSIG promoter 5′-ATGACCGGTTCCCGCCCCCT-3′; mut, end-labeled probes containing the mutant E2F1 binding site oligonucleotide 5′-ATGACCGGTACCCGCCCCCT-3′; competitor, unlabeled probes containing the E2F1 binding site; Ctrl, control.