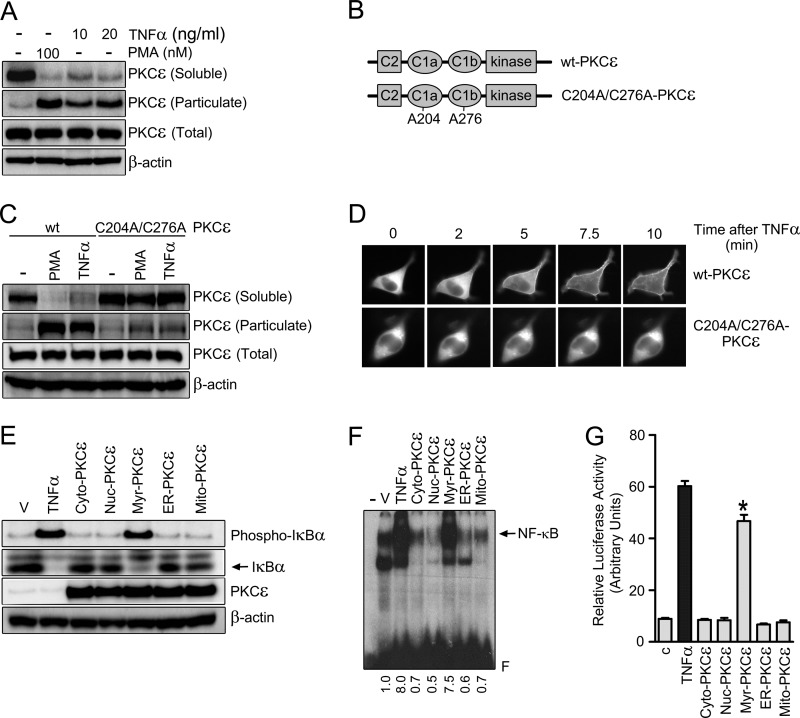
FIGURE 6.
PKCϵ translocation to the plasma membrane is required for TNFα-induced activation of NF-κB. A, LNCaP cells were treated for 30 min with either TNFα or PMA at the indicated concentrations and subject to fractionation into soluble and particulate fractions. Endogenous PKCϵ levels in each fraction were determined by Western blot analysis. B, schematic representation of WT and the C1a/C1b Cys-to-Ala PKCϵ mutant. C, LNCaP cells were transfected with pEGFP vectors encoding either the WT or C1 domain PKCϵ mutant. After 24 h, cells were treated with TNFα (20 ng/ml), PMA (100 nm), or vehicle for 30 min and subject to fractionation. GFP-PKCϵ levels in each fraction were determined by Western blot analysis using an anti-PKCϵ antibody. D, GFP-PKCϵ (WT or C1 domain mutant) localization in LNCaP cells was determined in response to TNFα (50 ng/ml) by real-time microscopy. E, LNCaP cells were transfected with different PKCϵ targeting expression vectors. After 24 h, cell extracts were prepared and subject to Western blot analysis for phosphorylated and total IκBα. F, NF-κB-DNA binding by EMSA was measured in nuclear cell extracts 24 h after transfection with the different constructs. V, vehicle. G, NF-κB luciferase reporter activity was measured 24 h after transfection of the different PKCϵ constructs. In E, F, and G, TNFα (10 ng/ml) was used as a positive control. Results are expressed as mean ± S.E. (n = 3). *, p < 0.05 versus control. In all cases, three independent experiments gave the similar results.