Abstract
Background:
Relapsing fever caused by Borrelia persica, is an acute tick-borne disease which is transmitted by soft ticks of Ornithodoros tholozani to human.
Methods:
Value of PCR and xenodiagnosis for detection of B. persica in O. tholozani ticks was compared. Sixty-four Borrelia-free ticks were fed on infected guinea pigs and used for the experiments. For xenodiagnosis, a group of 32 ticks in subsequent blood meal were fed on sterile guinea pigs and the indication of B. persica in the animal blood was tested 5–14 days later by dark-field microscopy. For PCR, all 64 ticks were subjected to PCR against B. persica rrs gene (16S-rDNA). Also sensitivity of PCR in terms of minimum detectable number of spirochetes as well as the effects of tick sex and post digestion was tested.
Results:
PCR revealed B. persica DNA in 98.4% ticks, in which B. persica were found in 25.0% by xenodiagnosis. PCR was enough sensitive to give positive results for DNA of 1 spirochete. PCR success rates were similar for male or female ticks. Course of time did not affect the efficacy of PCR and similar results were observed for ticks of immediately fed, semi- or completely gravid or completely digested blood ones.
Conclusion:
Our results indicate that due to very low specificity and time consuming, xenodiagnosis is not a useful method whereas PCR method has advantages for study the Borrelia prevalence in ticks.
Keywords: Borrelia persica, Ornithodoros tholozani, TBRF, Xenodiagnosis, PCR
Introduction
The tick borne relapsing fever (TBRF) is an acute infectious disease and important health problem in Middle East and Central Asia (1–5). The cause of this disease in Iran is Borrelia persica Dschunkowski 1912, that conveyed by the bite of the Ornithodoros tholozani soft tick. The natural route of tick infection is the feeding of a tick on an infected host during tick feeding in the next life stage (6). The ability of spirochetes to survive in the tick digestive tract, to migrate to hemolymph and then to the tick salivary glands, and to be transmitted with the saliva into the host skin are the crucial steps of pathogen transmission. Practical confirmation of spirochete infection in ticks in Iran has relied on xenodiagnosis method in which ticks are let to feed on guinea pigs followed by microscopic identification of the bacteria in blood smears of the animals. The bacteria should be searched for in the blood of guinea pigs daily from days 6 to 12 after the infection (7). Testing blood smear limited to a few days after inoculation and requires a highly experienced observer to identify the spirochetes and may necessitate a tedious and often unsuccessful microscopic search.
The development of the PCR has offered a new dimension in the diagnosis of infectious diseases. This method can amplify small amounts of DNA into millions of copies in less than 2–4 hours, and facilitates the sensitive and specific detection of DNA or RNA of pathogenic organisms (8). Usually the technique has been primarily used for detection of pathogens for which conventional diagnostic techniques are either too insensitive or too slow such as Borrelia spp (5). There are many research studies involving PCR in the diagnosis of Borrelia spp particularly B. burgdorferi infections in clinical samples (8–15).
Regarding the diagnostic performance of PCR with clinical specimens, the data so far available are variable. However, the diagnostic sensitivities of PCR for identification of B. persica in soft ticks have been neither systematically evaluated nor compared with other methods. In a recent but single paper, PCR was reported successfully to detect B. burgdorferi in hard ticks of Ixodes ricinus (6).
Since analysis and diagnostic PCR could be performed for both dead and alive ticks, and is not development stage dependent, it would be very advantageous and allows the study of different tick biological forms.
The aims of the present study were, by the use of laboratory experimentally infected animals, (i) to compare the diagnostic performance of PCR and xenodiagnosis in soft ticks of O. tholozani (ii) to investigate the sensitivity of PCR in terms of minimum detectable number of in vitro cultivated spirochetes (iii) to evaluate the diagnostic performance of PCR for male and female ticks and (iv) to assess the effect of post digestion on diagnostic performance of PCR. The results are expected to predict the efficacies of the methods as diagnostic tools for epidemiology of human TBRF.
Materials & Methods
Bacteria
The species of B. persica, strain Bijar isolated from O. tholozani in northwestern Iran was prepared from the Pasteur Institute of Iran. A stock of this bacterium is usually kept in the laboratory by culturing them in animal laboratory of guinea pigs.
Ticks
Laboratory strain of soft tick O. tholozani of the Argasidae family originated from the colony maintained at the insectarium of School of Public Health, Tehran University of Medical Sciences (SPH-TUMS). They were tested by PCR and microscopic methods for the presence of Borrelia spirochetes with negative results. Sixty four F1 adult ticks were selected and divided into two equal groups of 32 including 16 females and 16 males for xenodiagnosis and PCR experiments.
Guinea pigs
Pathogen-free guinea pigs (10 to 12-week-old females) were selected and maintained under standard conditions in the animal house unit at SPH-TUMS, Iran.
Experimental borreliosis in guinea pigs
We followed the protocol previously described by Lebech et al. (16) and experimental borreliosis were caused by intraperitoneally injection of 108 B. persica organisms to five pathogen-free guinea pigs.
Tick examination by Xenodiagnosis and PCR
On days 7–10 after inoculation, following confirmation of blood infection in microscopic field, the ticks of both groups were allowed to feed simultaneously at least 3 hours (until they fully blood engorged) on the infected guinea pigs to acquire Borrelia. The fed ticks were kept in the laboratory condition till full blood digestion. It normally took place at least 30 days in the laboratory condition. Then 32 ticks (first group) were stored in refrigerator for the following DNA isolation and PCR amplification. For xenodiagnosis method, 32 ticks (2nd group) subsequent of completely gravid with empty abdomen, were allowed to feed again on a pathogen-free guinea pig for next blood meal and on days 5, 7, 9, 11, and 14 post-infection, blood smear samples were prepared from their ears and examined by dark-field microscopy for growth of spirochetes. Moreover, this group of ticks was stored in refrigerator for further PCR analysis. Another 10 soft tick’s O. tholozani was used as the control group and were fed on a free pathogen guinea pig.
Isolation of DNA and PCR
The soft tissues of both groups of ticks were individually homogenized in PBS and used for DNA extraction. Genomic DNA was extracted with Bioneer Tissue DNA Spin Kit (Bioneer, South Korea), according to the manufacturer’s instructions. The rrs or 16S ribosomal RNA (16SrRNA) gene of Borrelia genome was amplified by PCR using forward primer Rec4 5′-ATGCTAGAAACTGCATGA-3′ and reverse primer Rec9 5′-TCGTCTGAGTCCCATCT-3′ as previously described (13). Amplicons of 523 bp were visualized on agarose 1% (w/v) gels stained with ethidium bromide. In brief, 5 μL of isolated DNA was amplified with the primers using initial denaturation of 3 min at 94°C followed by 35 cycles of 1 min at 93 °C, 1 min at 45°C, and 1 min at 72°C, followed by a 7-min hold at 72°C. PCR were performed in a volume of 25 μL including 10 pmol of each primer, Gelatin 0.1%, 1.25 units Taq DNA polymerase, 1.5mM MgCl2, 2.5 μL 10X buffer, 200 μM dNTPs, and 5 μL of DNA extracted from samples. To confirm the identity of Borrelia species used in this study, a few PCR products were sequenced (Seqlab, Germany) and compared to 16SrRNA genes sequences of other RF Borrelia spp available in the GenBank database using the BLAST and phylogenetic analysis online software embedded in Pubmed (http://www.ncbi.nlm.nih.gov/BLAST). The sensitivity of the PCR assay in terms of the minimum number of in vitro-cultivated B. persica cells was determined with spirochetal solutions containing 1–3, 4–6, 7–10 and more than ten Borrelia spirochetes per 10 μL soultion. The DNA of the 10 μL extracted was diluted in 5 μL ddH2O and used for PCR. To test the course of time on success of PCR, the DNA of second group of ticks were isolated in various times (4 specimens for each interval) after taking blood meal including one hour, and 1, 2, 4, 8, 16, 32 days (complete gravid), and after complete blood digestion (empty) when females laid eggs.
ddH2O, DNA of free pathogen lab strain of fed O. tholozani ticks, and blood DNA of none infected guinea pigs were used as negative controls. Also, blood DNA of the guinea pigs infected by inoculation was used as positive controls.
Results
Detection of B. persica
The result of utilities of PCR and xenodiagnosis methods has been shown in table 1. Borreli persica DNA was detectable by PCR in 61 out of 62 (98.4%) of ticks specimens that assumed to acquire B. persica spirochetes by feeding on the infected animals. A 523 bp PCR band was produced (Fig. 1) for all positive samples. In xenodiagnosis method, B. persica was found in the blood samples of only 8 out of 32 guinea pigs that infected ticks were fed on them, however, after full digestion of blood meal in the ticks, 23 out of theses 24 false negative ticks were PCR positive against the 16S-rDNA of B. persica (Table 1). To confirm the identity of the Borrelia species used in this study, the 16rDNA fragment of four PCR products were sequenced. These four samples had identical sequences and the consensus sequence was submitted to Genbank with the following accession number (U914141). The DNA sequences analyzed by BLAST was found to be 100% identical to that of isolates of B. persica from Iran (Genbank accession no. U42297). This confirmed that the RF spirochetes were used in this study was indeed B. persica.
Table 1:
Comparison of PCR and xenodiagnosis methods to measure contamination of the Borrelia persica in Ornithodoros tholozani ticks
| Method | Negative (%) | Positive (%) | Specimen | No tested |
|---|---|---|---|---|
| Xenodiagnosis (Microscopic) | 24 (75.0) | 8 (25.0) | Blood of Guinea pig | 32 |
| PCR following Xenodiagnosis | 1 (3.1) | 31 (96.9) | tick | 32 |
| PCR | 0 (0) | 32 (100) | tick | 32 |
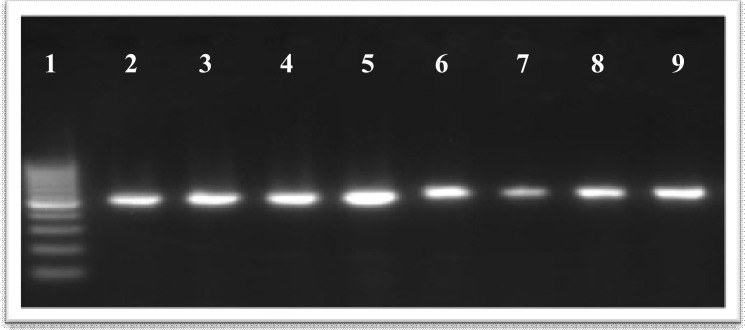
Fig. 1:
PCR amplification of 523 bp of 16S rDNA of Borrelia persica in infected Ornithodoros tholozani ticks and in guinea pig bloods. No. 1: 100 bp Molecular weight marker (Cinnagen, Iran), 2 and 3: respectively male and female ticks following xenodiagnosis and full blood digestion, 4–6: post blood meal respectively in ticks after one hour, 15 days, and >30 days (full blood digestion), 7–9: PCR products of animal blood when respectively 1, 5, and 10 spirochetes were added to the PCR mixture
Analytical sensitivity of the PCR assay in terms of the minimum number of in vitro-cultivated B. persica cells showed that a reproducible amplification can be achieved when one spirochete was added to the PCR mixture (Fig. 1).
The present PCR assay equally amplified B. persica DNAs from infected male and female ticks. In addition, post digestion or course of time had no effect on success of PCR and the present PCR assay equally amplified DNAs from samples either tested immediately or days after taking blood meal, fully gravid, or empty ones (Fig. 1).
Discussion
This is the first report showing advantages of PCR method, in comparison with xenodiagnosis method, for diagnosis of B. persica in soft ticks. The diagnosis of B. persica in ticks is often difficult due to the low number of spirochetes found in tick organs. The PCR assay had 97–100% sensitivity; hence it might be useful tool for the diagnosis of B. persica in human patients and other host reservoirs. In comparison with xenodiagnosis, PCR-based identification is also faster, is less expensive and requires less sample preparation. The xenodiagnosis method is extremely laborious, and considering the fact that the density of B. persicai in field ticks is even lower than that in experimentally infected ticks, the method is not useful in vector incrimination setting. The rate of infection detected by xenodiagnosis in this study was 25.0% which is comparable with 16% rate of infection detected by this method for detection of B. afzelii in Ixodes ricinus (L.) ticks (17).
Examination of ticks and other biological samples by culture on BSKH liquid medium followed by dark field microscopy is a reference standard for the demonstration of borrelial infection (12, 18). In this study we did not use this technique; however, this approach provides rather delayed results and is technically demanding and time consuming. In a study by Livesley et al. (19), the authors demonstrated that motile spirochaetes were not visible by dark-field microscopy in any of the cultures whereas 11/12 samples were PCR positive. Reller et al (20) designed a multiplex quantitative PCR (qPCR) assay to distinguish RF Borrelia from Plasmodium falciparum and P. vivax and specifically (100%) amplified pathogenic RF Borrelia (1 copy/reaction).
The 16S-rDNA is a highly conserved locus among the Borrelia spp and might be useful marker for detection of infection in ticks, animals, and patients infected with various RF Borrelia spp including B. persica, B. microti, B. baltazardi and B. latyschevii which are present in Central Asia and Middle-east countries (1, 3, 5). However, the initial PCR used in this study is not able to identify them to species level. However, there are a variety of PCR methods including qualitative (conventional PCR and nested PCR) or quantitative (competitive PCR and real-time PCR) which has been used for Borrelia detection and identification. Several factors including the gene target and primer set influence the effectiveness of a PCR assay. So far a number of genes including chromosomally carried genes such as rRNA genes (rrs), flaB, recA, GlpQ, and p66 and the plasmid-carried gene ospA have been employed in research laboratories (10–15). Among these, rrs and GlpQ have been used as targets for PCR analysis of B. persica (13–15). Aguero-Rosenfeld et al. (12) summarized the sensitivities and specificities of PCR assays for the detection of B. burgdorferi DNA in different clinical samples and showed a high range of sensitivities (36% to 100%) for different PCR assays.
In this study, xenodiagnosis method could not detect spirochetes in 75.0% of the animal blood. Although detailed biology of B. persica in guinea pig has not been properly studied yet, however, this low sensitivity is not in accordance with the result of another study showed that a single spirochete of B. turicatae, B. hermsii, or B. duttonii is sufficient to produce infection of laboratory animals and will appear in the blood as soon as 1 h after intraperitoneal inoculation of a large inoculum (21–23). It is shown that following presence of spirochetes in the blood, they multiply as often as once every 6 h and in susceptible animals, there may be as many as 10,000,000 spirochetes per ml of blood during peak spirochetemias (22). The absence of detectable spirochetes by microscopy in most of the animal blood of this study could be due to several reasons. It is shown that the transmission pathway employed to infect the host and the duration of systemic infection are important factors responsible for establishment or persistence of tick-borne pathogens in a given tick-host system (23).
One might suggest that due to long passaged in animals, they may lose the ability to infect and be transmitted by ticks (24–25). In one such strain of B. duttonii that was examined, the spirochetes were able to persist in the mid gut of O. moubata after feeding, but they could not enter the hemocoel (26). However, when the B. duttonii organisms were injected directly into the hemolymph in the hemocoel, ticks were then capable of transmitting the spirochetes to mice (27). It appears that the mouse-passaged borrelial variants were no longer capable of penetrating the tick’s midgut wall (2). Recently, it is shown that there is a very early effect of tick saliva on the proliferation and distribution of Borrelia spirochetes in the host, probably due to the effect of saliva on the host innate immunity mechanisms (6). In contrast, long-term animal passage need not always result in loss of the ability to be transmitted by ticks; a strain of B. hispanica was passed 71 times in guinea pigs and yet remained tick transmissible (27). The B. persica strain we used in this study usually, at least twice per year, is transmitted to bite of ticks on infected guinea pigs to keep its ability to infect ticks. One possible explanation for low rate of infection in xenodiagnosis method might be related to the environmental conditions and their effects on the aggregation of Borrelia spirochetes in ticks. It has been documented that Lyme borreliosis agents form aggregates during tick blood feeding, when they get transmitted from hard tick vector to mammalian hosts. The Borrelia aggregations are temperature, pH, and growth phase dependent and environmental conditions such high temperature, low pH, and high cell density favorable for their aggregation (28). However, favorable condition for B. persica in soft ticks is not or poorly characterized.
Reznik (29) assessed efficiency of B. persica transmission by O. papillipes ticks and assumed two forms (mild and typical) of borreliemia in guinea pigs caused by ticks’ bites. The mild form is not easily diagnosed by the analysis of animal blood preparations, being encountered in 40% of cases. More investigation needs to clarify which form of borreliemia in guinea pigs has been occurred in this study.
Here we showed that PCR can equally amplify the DNA of B. persica in male and female and in immediately fed or days after taking blood meal even after completely empty abdomen ticks. This is important in epidemiological studies regarding to vector incrimination of tick borne diseases. Many studies have shown that male and female ticks are not equivalently infected by Borrelia spp and female ticks are more often infected than male ticks and thus transmit this pathogenic agent more often (30–33). The average number of spirochetes in female ticks is greater than males (6).
Diagnostic value of PCR for detection of B. burgdorferi DNA using 16S ribosomal RNA in clinical specimens from patients with erythema migrans (EM) and Lyme neuroborreliosis was tested and compared with those obtained by in vitro culture and serological testing (33). These authors showed that PCR of skin biopsy specimens is currently the most sensitive and specific test for the diagnosis of patients with EM, superior to culture and serological testing. In conclusion, the 16S-rDNA PCR was found as a highly reliable assay for the diagnosis of B. persica. However, its utility should be tested for the other Borrelia spp (B. microti, B. baltazardi and B. latyschevii) in the region. This rapid assay could facilitate the diagnosis of B. persica in different biological samples and provide an important tool for better understanding this yet poorly characterized spirochete.
Ethical Considerations
Ethical issue principles including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc. have been completely observed by the authors.
Acknowledgments
This work was supported by a research grant from Tehran University of Medical Sciences (TUMS) (Grant no. 86-03-27-6268) to M.A. Oshaghi. We thank Abolhassani, Eskandari, and Hosseni for their perfect technical assistance. The authors declare that there is no conflict of interests.
References
- 1.Karimi Y, Hovdn-Houqen K, Bricg-Andersen A, Asmar M. Borrelia persica and B. baltazarid SP. Nov. Experimental pathogenicity for some animal and comparison of the ultrastructure. Ann Microbiol (Paris) 1979;130:157–68. [PubMed] [Google Scholar]
- 2.Karimi Y. Relapsing Fever and its Epidemiology. Tehran: Pasteur Institute of Iran; 1981. [Google Scholar]
- 3.Barbour AG, Hayes SF. Biology of Borrelia Species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arshi S, Majidpoor A, Sadeghi H, Asmar M, Emadi D, Derakhshan MH. Relapsing fever in Ardebil, a northwestern province of Iran. Arch Iranian Med. 2002;5:141–45. [Google Scholar]
- 5.Barmaki A, Rafinejad J, Vatandoost H, Telmadarraiy Z, Mohtarami F, Leghaei SH, Oshaghi MA. Study on Presence of Borrelia persica in Soft Ticks in Western Iran. Iran J Arthropod-Bore Dis. 2010;4:19–25. [PMC free article] [PubMed] [Google Scholar]
- 6.Horka H, Cerna-Kyckova K, Fiserova L, Kopecky G. Efficiency of experimental infection of Ixodes ricinus ticks with Borrelia burgdorferi spirochetes. Int J Med Microbiol. 2008;298:177–79. [Google Scholar]
- 7.Reznik EP. An experimental quantitative assessment of the efficiency of Borrelia persica transmission by Ornithodoros papillipes ticks. 1. 2 forms of borreliemia in guinea pigs. Med Parazitol (Mosk) 1990;3:17–21. [PubMed] [Google Scholar]
- 8.Schmidt BL. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin Microbiol Rev. 1997;10:185–201. doi: 10.1128/cmr.10.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz I, Varde S, Nadelman RB, Wormser GP, Fish D. Inhibition of Efficient Polymerase Chain Reaction Amplification of Borrelia burgdorferi DNA in Blood-Fed Ticks. Am J Trop Med Hyg. 1997;56:339–42. doi: 10.4269/ajtmh.1997.56.339. [DOI] [PubMed] [Google Scholar]
- 10.Cerar T, Ogrinc K, Cimperman J, Lotri-Furlan S, Strle F, Ruzic-Sabljic E. Validation of Cultivation and PCR Methods for Diagnosis of Lyme Neuroborreliosis. J Clin Microbiol. 2008;46:3375–79. doi: 10.1128/JCM.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assous MV, Wilamowski A. Relapsing fever borreliosis in Eurasia--forgotten, but certainly not gone. Clin Microbiol Infect. 2009;15:407–14. doi: 10.1111/j.1469-0691.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- 12.Aguero-Rosenfeld ME, Wang G, Schwartz Ira, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ras NM, Lascola B, Postic D, Culter SJ, Rodhain F. Phylogenesis of relapsing fever Borrelia spp. Int J Sys Bacteriol. 1996;46:859–65. doi: 10.1099/00207713-46-4-859. [DOI] [PubMed] [Google Scholar]
- 14.Halperin T, Orr N, Cohen R, Hasin T, Davidovitch N, Klement E, et al. Detection of relapsing fever in human blood samples from Israel using PCR targeting the glycerophosphodiester phosphodiesterase (GlpQ) gene. Acta Trop. 2006;98:189–95. doi: 10.1016/j.actatropica.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Oshaghi MA, Rafinejad J, Choubdar N, Piazak N, Vatandoost H, Telmadarraiy Z, et al. Discrimination of Relapsing Fever Borrelia persica and B. microtti by Diagnostic Species-Specific Primers and PCR-Restriction Fragment Length Polymorphism. Vector Borne Zoonotic Dis. 2011;11(3):201–07. doi: 10.1089/vbz.2009.0170. [DOI] [PubMed] [Google Scholar]
- 16.Lebech AM, Clemmensen O, Hansen K. Comparison of in vitro culture, immunohistochemical staining, and PCR for detection of Borrelia burgdorferi in tissue from experimentally infected animals. J Clin Microbiol. 1995;33:2328–33. doi: 10.1128/jcm.33.9.2328-2333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiserov L, Cern K, Hork H, Kopeck J. Two ways of experimental infection of Ixodes ricinus ticks (Acari: Ixodidae) with spirochetes of Borrelia burgdorferi sensu lato complex. Folia Parasitol (Praha) 2008;55:150–54. [PubMed] [Google Scholar]
- 18.Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;362:1639–47. doi: 10.1016/S0140-6736(03)14798-8. [DOI] [PubMed] [Google Scholar]
- 19.Livesley MA, Carey D, Gern L, Nuttall PA. Problems of isolating Borrelia burgdorferi from ticks collected in United Kingdom foci of Lyme disease. Med Vet Entomol. 1994;8:172–78. doi: 10.1111/j.1365-2915.1994.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 20.Reller ME, Clemens EG, Schachterle SE, Mtove GA, Sullivan DJ, Dumler JS. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. J Clin Microbiol. 2011;49(9):3245–49. doi: 10.1128/JCM.00940-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuhardt VT, Wilkerson M. Relapse phenomena in rats infected with single spirochetes (Borrelia recurrentis var. turicatae) J Bacteriol. 1951;62:215–19. doi: 10.1128/jb.62.2.215-219.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoenner HG, Dodd T, Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982;156:1297–311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison A, Montgomery WI, Bown KJ. Investigating the persistence of tick-borne pathogens via the R0 model. Parasitology. 2011;26:1–10. doi: 10.1017/S0031182011000400. [DOI] [PubMed] [Google Scholar]
- 24.Brumpt E. Essai de transmission par l’Ornithodorus turicata, d’une souche de Spirochaet anovyi, ayant subi plus de 3,000 passages sur rats. C R Soc Biol. 1934;115:600–602. [Google Scholar]
- 25.Grfn H. Die experimentelle Ubertragung von Ruckfal-Ifieber-Spirochate durch Ornithodorus moubata. Z Hyg Infektionskr. 1950;131:198–218. [PubMed] [Google Scholar]
- 26.Varma MGR. Infections of Ornithodoros ticks with relapsing fever spirochetes and the mechanisms of their transmission. Ann Trop Med Parasitol. 1956;50:18–31. doi: 10.1080/00034983.1956.11685735. [DOI] [PubMed] [Google Scholar]
- 27.Colas-Belcour J, Verrent G. Transmissibilite et virulence d’une souche de Spirochaeta hispanica. Bull Soc Pathol Exot. 1949;42:470–79. [Google Scholar]
- 28.Srivastava SY, de Silva AM. Characterization of Borrelia burgdorferi Aggregates. Vector Borne Zoonotic Dis. 2009;9:323–29. doi: 10.1089/vbz.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reznik EP. [An experimental quantitative assessment of the efficiency of Borrelia persica transmission by Ornithodoros papillipes ticks. 1. 2 forms of borreliemia in guinea pigs] Med Parazitol (Mosk) 1990;3:17–21. [Article in Russian]. [PubMed] [Google Scholar]
- 30.Skotarczak B. Isolation of Borrelia burgdorferi sensu lato in ticks Ixodes ricinus by polymerase chain reaction (PCR) Wiad Parazytol. 2000;46:93–99. [PubMed] [Google Scholar]
- 31.De Meeûs T, Lorimier Y, Renaud F. Lyme borreliosis agents and the genetics and sex of their vector, Ixodes ricinus. Microbes Infect. 2004;6:299–304. doi: 10.1016/j.micinf.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Cisak E, Wojcik-Fatla A, Stojek N, Chmielewska-Badora J, Zwolinski J, Buczek A, Dutkiewicz J. Prevalence of Borrelia burgdorferi genospecies in Ixodes ricinus ticks from Lublin region (eastern Poland) Ann Agric Environ Med. 2006;13:301–06. [PubMed] [Google Scholar]
- 33.Lebech AM, Hansen K, Brandrup F, Clemmensen O, Halkier-Sørensen L. Diagnostic value of PCR for detection of Borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and Lyme neuroborreliosis. Mol Diagn. 2000;5:139–50. doi: 10.1007/BF03262032. [DOI] [PubMed] [Google Scholar]