Figure 8.
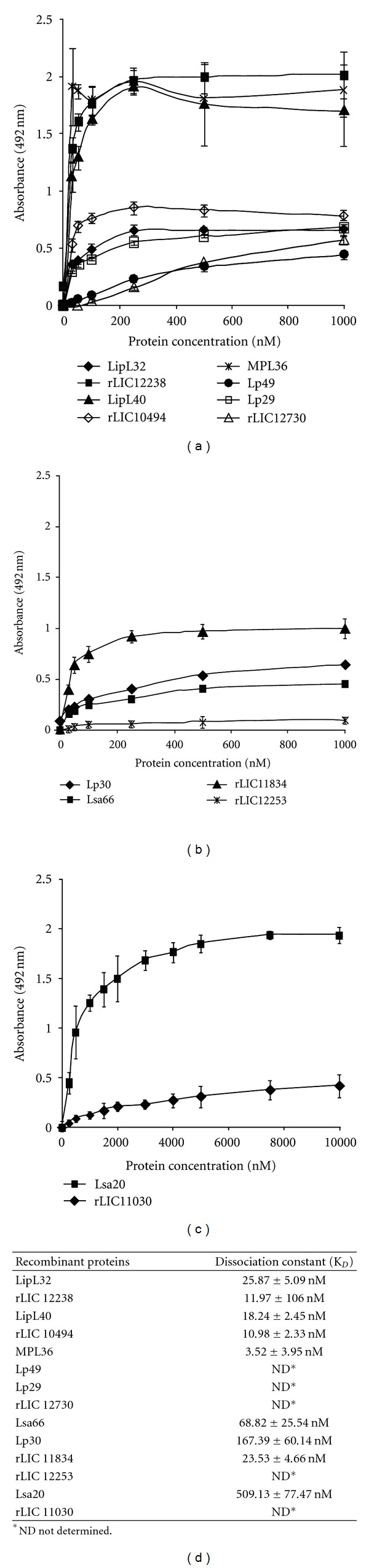
Characterization of recombinant proteins binding to PLG. In (a),(b), and (c) PLG (10 μg/mL) was immobilized in 96-well ELISA plates, and each recombinant protein at 0 to 1,000 nM was added for interaction. The binding was detected using antiserum raised in mice against each protein at appropriate dilutions (1 : 4,000 for LipL32; 1 : 5,000 for rLIC12238, LipL40, and MPL36; 1 : 1,000 for Lp29, Lp49, Lsa20, and rLIC11030; 1 : 500 for rLIC12730, Lsa66, and Lp30; 1 : 750 for rLIC11834 and rLIC12253), followed byhorseradish peroxidase-conjugated anti-mouse IgG. Data represent the mean absorbance values ± the standard deviation of six replicates for each experimental group. The results are representative of two independent experiments. In (d) The dissociation constant (KD) was calculated based on ELISA data for the recombinant proteins that reached equilibrium up to a concentration of 1,000 nM.
