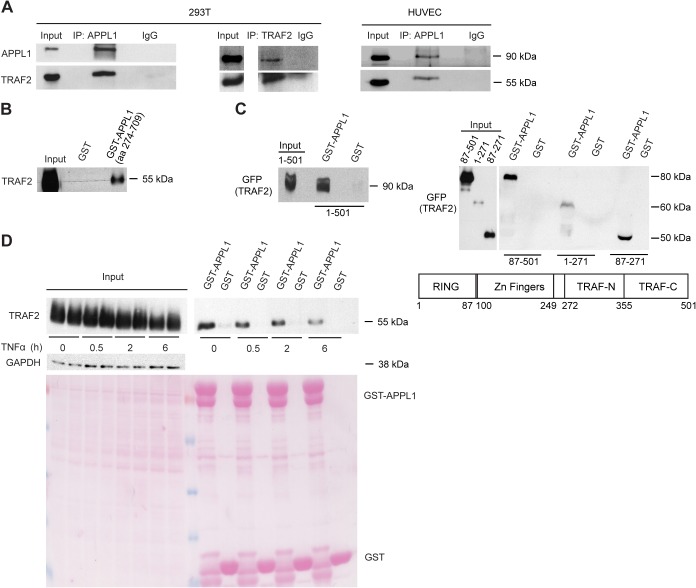
Fig. 1.
APPL1 interacts with TRAF2. (A) Endogenous APPL1 and TRAF2 interact in HEK293T and HUVEC cells. Cell lysates from untransfected HEK293T and HUVEC cells were subjected to coimmunoprecipitation with either rabbit anti-APPL1 antiserum or anti-TRAF2 mouse monoclonal antibody. Rabbit and mouse IgG were used as negative controls, respectively. Input represents 10% of total cell lysate used for immunoprecipitation. (B) APPL1 and TRAF2 interact directly. An in vitro translated TRAF2 was incubated with GST alone or APPL1 (a.a. 274–709) fused with GST. The protein complexes were analyzed by western blotting with anti-TRAF2 monoclonal antibody. Input shows 5% of total in vitro translated TRAF2. (C) The zinc finger region of TRAF2 binds to the PTB domain of APPL1. HEK293T cells were transfected with GFP-tagged TRAF2 (full length a.a. 1–501, left panel; or a.a. 87–501, 1–271, 87–271, right panel) 24 h prior to lysis. Cell lysates were incubated with GST alone or GST–APPL1 (a.a. 429–709). GFP–TRAF2 bound to APPL1 was detected with anti-GFP antibody. Input shows 10% of total cell lysate. (D) APPL1–TRAF2 interaction is regulated by TNFα. HEK293T cells were transfected with TRAF2 expression vector and, after 24 h, were stimulated with TNFα for 0.5, 2, 6 h or left untreated. Cell lysates were subjected to pull-down experiments with GST alone or GST–APPL1 (a.a. 274–709) and processed as in B (upper panel). Input shows 10% of total cell lysate. GAPDH level and Ponceau S staining (lower panel) serve as loading controls.