Fig. 3.
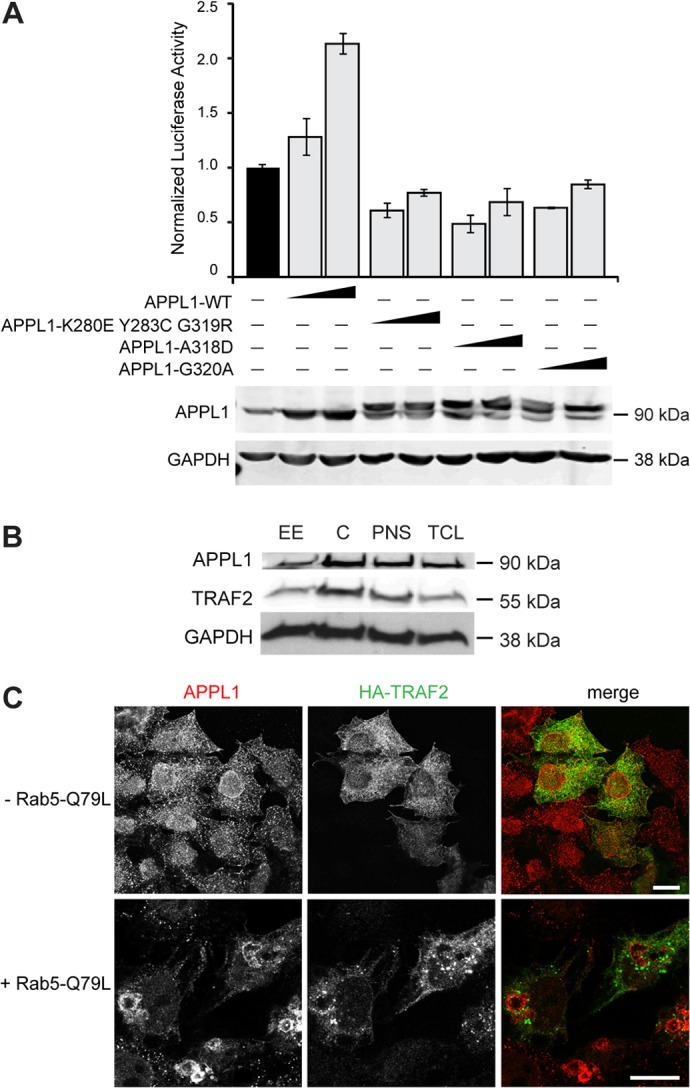
Endosomal recruitment of APPL1 is necessary for its function in the NF-κB pathway. (A) APPL1 endosomal localization is necessary for NF-κB activation. HEK293T cells were cotransfected with reporter vectors together with 0.5 and 1 µg of untagged wild-type APPL1 or APPL1 mutants unable to bind Rab5 (myc-APPL1-K280E,Y283C,G319R and myc-APPL1-A318D) or Rab21 (myc-APPL1-G320A), and measurements of the luciferase activity were made (upper panel). The overexpression levels of APPL1 variants were tested by immunoblotting with anti-APPL1 antibody (lower panel). (B) TRAF2 is localized on endosomal membranes. Early endosomal (EE) and cytoplasmic (C) fractions, postnuclear supernatant (PNS) and total cell lysate (TCL) from HeLa cells were analyzed by western blotting with anti-APPL1, -TRAF2 and -GAPDH (loading control). (C) TRAF2 is not recruited to the enlarged APPL1-positive endosomes. Twenty-four hours after transfection with HA–TRAF2 alone (upper panel) or in combination with Rab5-Q79L (bottom panel), HeLa cells were fixed and stained with antibodies against APPL1 (red) and HA (green). Scale bars: 20 µm.
