Fig. 7.
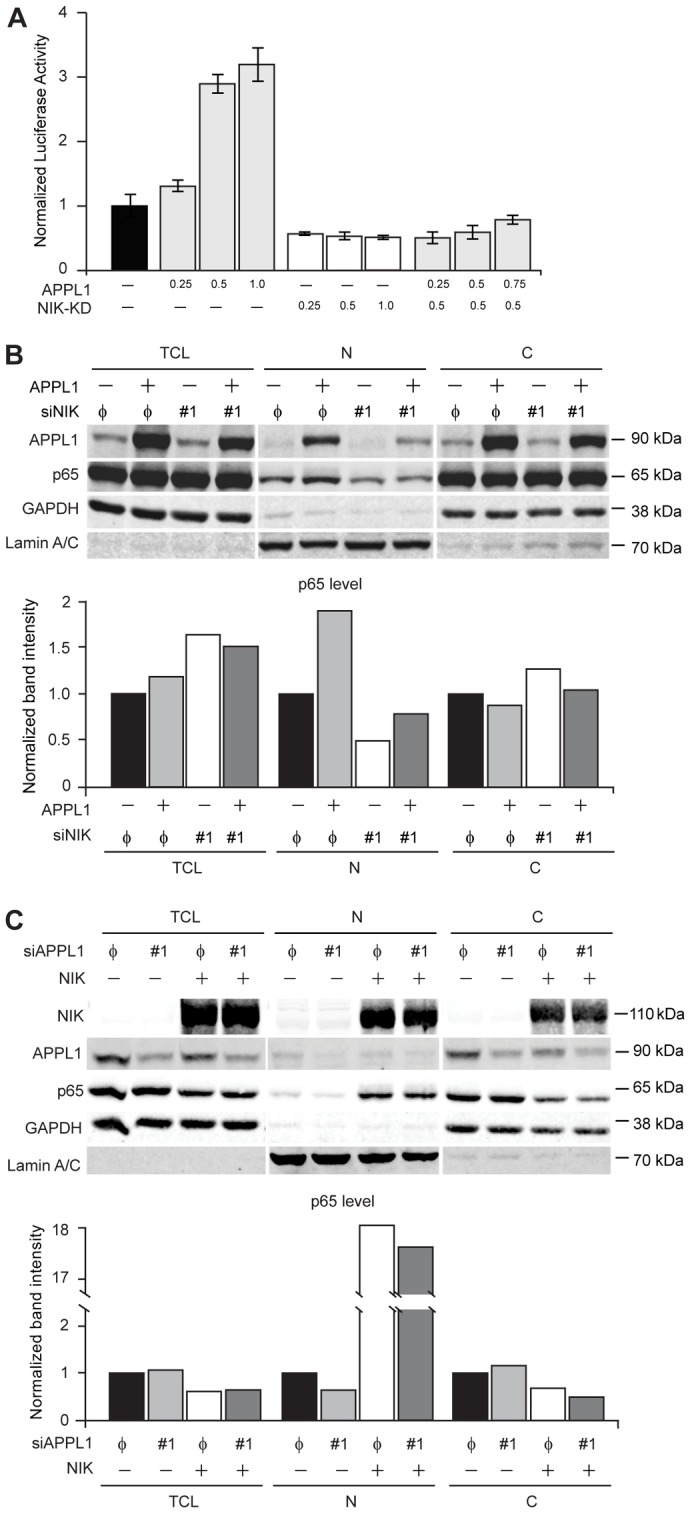
APPL1-induced NIK stability activates the canonical NF-κB pathway. (A) A kinase-deficient mutant of NIK abolishes APPL1-induced activation of the NF-κB reporter. HEK293T cells were cotransfected with reporter plasmids along with the different combinations of APPL1 and NIK-KD expression vectors, as indicated (all values are in µg of DNA). The luciferase reporter activity was measured as described in the Materials and Methods. (B) APPL1 overexpression does not trigger nuclear translocation of p65 upon NIK depletion. HEK293T cells were transfected with APPL1-expressing vector or siRNAs (non-targeting φ or NIK #1; efficiency of NIK knockdown was verified by RT-PCR). Forty-eight hours after transfection total cell lysate (TCL), cytoplasmic (C) and nuclear (N) fractions were analyzed by western blotting with anti-APPL1, -p65, -GAPDH and -lamin-A/C antibodies (upper panel) and the intensity of p65 band was quantified (lower panel). (C) NIK overexpression enhances nuclear localization of p65 upon knockdown of APPL1. HEK293T cells were transfected with NIK-expressing vector or siRNAs (non-targeting φ or APPL1 #1). Forty-eight hours after transfection, the cell fractions were prepared and analyzed as in B. Representative results from three independent experiments are shown in B and C.
